
Hematite, also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of Fe
2O
3. It has the same crystal structure as corundum (Al
2O
3) and ilmenite (FeTiO
3). With this it forms a complete solid solution at temperatures above 950 °C (1,740 °F).

Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation. Sedimentation is the collective name for processes that cause these particles to settle in place. The particles that form a sedimentary rock are called sediment, and may be composed of geological detritus (minerals) or biological detritus. The geological detritus originated from weathering and erosion of existing rocks, or from the solidification of molten lava blobs erupted by volcanoes. The geological detritus is transported to the place of deposition by water, wind, ice or mass movement, which are called agents of denudation. Biological detritus was formed by bodies and parts of dead aquatic organisms, as well as their fecal mass, suspended in water and slowly piling up on the floor of water bodies. Sedimentation may also occur as dissolved minerals precipitate from water solution.

Banded iron formations are distinctive units of sedimentary rock consisting of alternating layers of iron oxides and iron-poor chert. They can be up to several hundred meters in thickness and extend laterally for several hundred kilometers. Almost all of these formations are of Precambrian age and are thought to record the oxygenation of the Earth's oceans. Some of the Earth's oldest rock formations, which formed about 3,700 million years ago (Ma), are associated with banded iron formations.

Goethite is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α-polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient times for its use as a pigment. Evidence has been found of its use in paint pigment samples taken from the caves of Lascaux in France. It was first described in 1806 based on samples found in the Hollertszug Mine in Herdorf, Germany. The mineral was named after the German polymath and poet Johann Wolfgang von Goethe (1749–1832).

Chert is a hard, fine-grained sedimentary rock composed of microcrystalline or cryptocrystalline quartz, the mineral form of silicon dioxide (SiO2). Chert is characteristically of biological origin, but may also occur inorganically as a chemical precipitate or a diagenetic replacement, as in petrified wood.

Diagenesis is the process that describes physical and chemical changes in sediments first caused by water-rock interactions, microbial activity, and compaction after their deposition. Increased pressure and temperature only start to play a role as sediments become buried much deeper in the Earth's crust. In the early stages, the transformation of poorly consolidated sediments into sedimentary rock (lithification) is simply accompanied by a reduction in porosity and water expulsion, while their main mineralogical assemblages remain unaltered. As the rock is carried deeper by further deposition above, its organic content is progressively transformed into kerogens and bitumens.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, since rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as hydrous ferric oxide.

Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.

A concretion is a hard, compact mass formed by the precipitation of mineral cement within the spaces between particles, and is found in sedimentary rock or soil. Concretions are often ovoid or spherical in shape, although irregular shapes also occur. The word 'concretion' is derived from the Latin concretio "(act of) compacting, condensing, congealing, uniting", itself from con meaning "together" and crescere meaning "to grow".
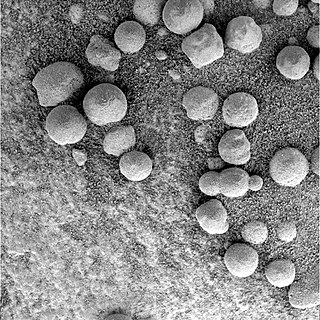
Martian spherules (also known as hematite spherules, blueberries, & Martian blueberries) are small spherules (roughly spherical pebbles) that are rich in an iron oxide (grey hematite, α-Fe2O3) and are found at Meridiani Planum (a large plain on Mars) in exceedingly large numbers.

Maghemite (Fe2O3, γ-Fe2O3) is a member of the family of iron oxides. It has the same formula as hematite, but the same spinel ferrite structure as magnetite (Fe3O4) and is also ferrimagnetic. It is sometimes spelled as "maghaemite".

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).

Various theories of ore genesis explain how the various types of mineral deposits form within Earth's crust. Ore-genesis theories vary depending on the mineral or commodity examined.

The Navajo Sandstone is a geological formation in the Glen Canyon Group that is spread across the U.S. states of southern Nevada, northern Arizona, northwest Colorado, and Utah as part of the Colorado Plateau province of the United States.

Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydrothermal hot springs and scales, soils, and areas affected by mining. It can be precipitated directly from oxygenated iron-rich aqueous solutions, or by bacteria either as a result of a metabolic activity or passive sorption of dissolved iron followed by nucleation reactions. Ferrihydrite also occurs in the core of the ferritin protein from many living organisms, for the purpose of intra-cellular iron storage.

The surface color of the planet Mars appears reddish from a distance because of rusty atmospheric dust. From close up, it looks more of a butterscotch, and other common surface colors include golden, brown, tan, and greenish, depending on minerals.
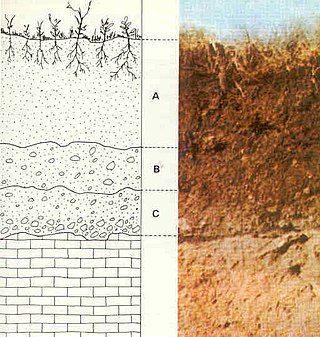
Saprolite is a chemically weathered rock. Saprolites form in the lower zones of soil profiles and represent deep weathering of the bedrock surface. In most outcrops, its color comes from ferric compounds. Deeply weathered profiles are widespread on the continental landmasses between latitudes 35°N and 35°S.

Iron-rich sedimentary rocks are sedimentary rocks which contain 15 wt.% or more iron. However, most sedimentary rocks contain iron in varying degrees. The majority of these rocks were deposited during specific geologic time periods: The Precambrian, the early Paleozoic, and the middle to late Mesozoic. Overall, they make up a very small portion of the total sedimentary record.

The geology of Luxembourg is divided into two geologic regions: Rheinisches Schiefergeblige in the north, extending into the Ardennes region in Belgium, and the Oesling Zone to the north of Ettelbruck. The country is underlain by the Hercynian orogeny related Givonne Anticlinorium, which mainly contains Early Devonian sandstone and shale. Rocks closer to the surface are primarily from the Cretaceous and are cut by the Sauer River and its tributaries.
Iron ochre or iron ocher (Ancient Greek: ὠχρός, pale yellow, orange) — at least three iron ore minerals, common abrasives and pigments with a red-brown or brown-orange hue and the powdery consistency of ocher, were known under such a trivial name. The term “iron ocher” was primarily used among mineral collectors, geologists, miners and representatives of related craft professions. It may refer to:






















