Nitrogen fixation is a chemical process by which molecular nitrogen (N
2), with a strong triple covalent bond, in the air is converted into ammonia (NH
3) or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmospheric nitrogen is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or diazotrophy is an important microbially mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif).

The green sulfur bacteria are a phylum of obligately anaerobic photoautotrophic bacteria that metabolize sulfur.

Heterocysts or heterocytes are specialized nitrogen-fixing cells formed during nitrogen starvation by some filamentous cyanobacteria, such as Nostoc punctiforme, Cylindrospermum stagnale, and Anabaena sphaerica. They fix nitrogen from dinitrogen (N2) in the air using the enzyme nitrogenase, in order to provide the cells in the filament with nitrogen for biosynthesis.
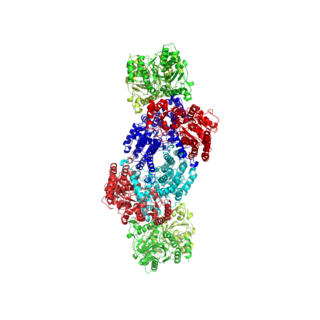
Nitrogenases are enzymes (EC 1.18.6.1EC 1.19.6.1) that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase.

Purple bacteria or purple photosynthetic bacteria are Gram-negative They are pigmented with bacteriochlorophyll a or b, together with various carotenoids, which give them colours ranging between purple, red, brown, and orange. They may be divided into two groups – purple sulfur bacteria and purple non-sulfur bacteria (Rhodospirillaceae). Purple bacteria are anoxygenic phototrophs widely spread in nature, but especially in aquatic environments, where there are anoxic conditions that favor the synthesis of their pigments.
The Chromatiaceae are one of the two families of purple sulfur bacteria, together with the Ectothiorhodospiraceae. They belong to the order Chromatiales of the class Gammaproteobacteria, which is composed by unicellular Gram-negative organisms. Most of the species are photolithoautotrophs and conduct an anoxygenic photosynthesis, but there are also representatives capable of growing under dark and/or microaerobic conditions as either chemolithoautotrophs or chemoorganoheterotrophs.

Azotobacter is a genus of usually motile, oval or spherical bacteria that form thick-walled cysts and may produce large quantities of capsular slime. They are aerobic, free-living soil microbes that play an important role in the nitrogen cycle in nature, binding atmospheric nitrogen, which is inaccessible to plants, and releasing it in the form of ammonium ions into the soil. In addition to being a model organism for studying diazotrophs, it is used by humans for the production of biofertilizers, food additives, and some biopolymers. The first representative of the genus, Azotobacter chroococcum, was discovered and described in 1901 by Dutch microbiologist and botanist Martinus Beijerinck. Azotobacter species are Gram-negative bacteria found in neutral and alkaline soils, in water, and in association with some plants.
Azotobacter vinelandii is Gram-negative diazotroph that can fix nitrogen while grown aerobically. These bacteria are easily cultured and grown.

Glutamine synthetase (GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine:
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
The nif genes are genes encoding enzymes involved in the fixation of atmospheric nitrogen into a form of nitrogen available to living organisms. The primary enzyme encoded by the nif genes is the nitrogenase complex which is in charge of converting atmospheric nitrogen (N2) to other nitrogen forms such as ammonia which the organism can use for various purposes. Besides the nitrogenase enzyme, the nif genes also encode a number of regulatory proteins involved in nitrogen fixation. The nif genes are found in both free-living nitrogen-fixing bacteria and in symbiotic bacteria associated with various plants. The expression of the nif genes is induced as a response to low concentrations of fixed nitrogen and oxygen concentrations (the low oxygen concentrations are actively maintained in the root environment of host plants). The first Rhizobium genes for nitrogen fixation (nif) and for nodulation (nod) were cloned in the early 1980s by Gary Ruvkun and Sharon R. Long in Frederick M. Ausubel's laboratory.
Rhodobacter sphaeroides is a kind of purple bacterium; a group of bacteria that can obtain energy through photosynthesis. Its best growth conditions are anaerobic phototrophy and aerobic chemoheterotrophy in the absence of light. R. sphaeroides is also able to fix nitrogen. It is remarkably metabolically diverse, as it is able to grow heterotrophically via fermentation and aerobic and anaerobic respiration. Such a metabolic versatility has motivated the investigation of R. sphaeroides as microbial cell factory for biotechnological applications.
Rhodopseudomonas palustris is a rod-shaped, Gram-negative purple nonsulfur bacterium, notable for its ability to switch between four different modes of metabolism.
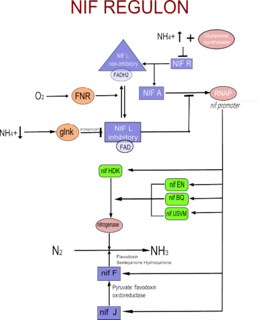
The Nif regulon is a set of seven operons used to regulate nitrogen fixation in the coliform bacterium Klebsiella pneumoniae under anaerobic and microaerophilic conditions. It includes 17 nif genes, and is situated between the his and the Shi-A operon of the bacterium.

The PII family comprises a group of widely distributed signal transduction proteins found in nearly all Bacteria and also present in Archaea and in the chloroplasts of Algae and plants. PII form barrel-like homotrimers with a flexible loop, namely T-loop, emerging from each subunit. PII proteins have extraordinary sensory properties; they can exist in a vast range of structural status accordingly to the levels of ATP, ADP and 2-oxogluratate. These metabolites interact allosterically with PII in three conserved binding sites located in the lateral cavity between each PII subunit. ATP and ADP bind competitively to the nucleotide binding whereas the 2-oxoglutarate only interacts with PII in the presence of MgATP.
Chlorobaculum tepidum, previously known as Chlorobium tepidum, is an anaerobic, thermophilic green sulfur bacteria first isolated from New Zealand. Cells are gram-negative and non-motile rods of variable length. They contain bacteriochlorophyll c and chlorosomes.
The glnALG operon is an operon that regulates the nitrogen content of a cell. It codes for the structural gene glnA the two regulatory genes glnL and glnG. glnA encodes glutamine synthetase, an enzyme which catalyzes the conversion of glutamate and ammonia to glutamine, thereby controlling the nitrogen level in the cell. glnG encodes NRI which regulates the expression of the glnALG operon at three promoters, which are glnAp1, glnAp2 located upstream of glnA) and glnLp. glnL encodes NRII which regulates the activity of NRI. No significant homology is found in Eukaryotes.

Cyanothece is a genus of unicellular, diazotrophic, oxygenic photosynthesizing cyanobacteria.
Rhodobacter capsulatus is a species of purple bacteria, a group of bacteria that can obtain energy through photosynthesis. Its name is derived from the Latin adjective "capsulatus", itself derived Latin noun "capsula", and the associated Latin suffix for masculine nouns, "-atus".

Crocosphaera watsonii is an isolate of a species of unicellular, diazotrophic marine cyanobacteria which represent less than 0.1% of the marine microbial population. They thrive in offshore, open-ocean oligotrophic regions where the waters are warmer than 24 degrees Celsius. Crocosphaera watsonii cell density can exceed 1,000 cells per milliliter within the euphotic zone; however, their growth may be limited by the concentration of phosphorus. Crocosphaera watsonii are able to contribute to the oceanic carbon and nitrogen budgets in tropical oceans due to their size, abundance, and rapid growth rate. Crocosphaera watsonii are unicellular nitrogen fixers that fix atmospheric nitrogen to ammonia during the night and contribute to new nitrogen in the oceans. They are a major source of nitrogen to open-ocean systems. Nitrogen fixation is important in the oceans as it not only allows phytoplankton to continue growing when nitrogen and ammonium are in very low supply but it also replenishes other forms of nitrogen, thus fertilizing the ocean and allowing more phytoplankton growth.












