
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other, nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD+ and NADH (H for hydrogen), respectively.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
In enzymology, a cholest-5-ene-3β,7α-diol 3β-dehydrogenase (EC 1.1.1.181) is an enzyme that catalyzes the chemical reaction
In enzymology, a galactose 1-dehydrogenase (EC 1.1.1.48) is an enzyme that catalyzes the chemical reaction
In enzymology, a sorbose 5-dehydrogenase (NADP+) (EC 1.1.1.123) is an enzyme that catalyzes the chemical reaction
In enzymology, a testosterone 17beta-dehydrogenase is an enzyme that catalyzes the chemical reaction between testosterone and androst-4-ene-3,17-dione. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor.

In enzymology, a 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) is an enzyme that catalyzes the chemical reaction
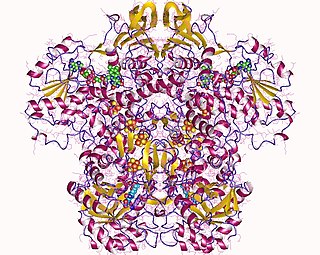
In enzymology, a dihydropyrimidine dehydrogenase (NADP+) (EC 1.3.1.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a L-aminoadipate-semialdehyde dehydrogenase (EC 1.2.1.31) is an enzyme that catalyzes the chemical reaction
CDP-4-dehydro-6-deoxyglucose reductase (EC 1.17.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a rubredoxin-NAD+ reductase (EC 1.18.1.1) is an enzyme that catalyzes the chemical reaction.
Azobenzene reductase also known as azoreductase (EC 1.7.1.6) is an enzyme that catalyzes the chemical reaction:
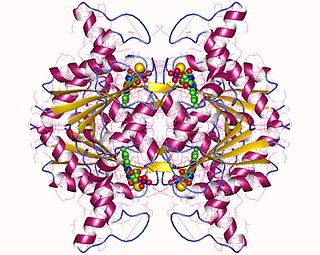
In enzymology, a NAD(P)H dehydrogenase (quinone) (EC 1.6.5.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a NADPH dehydrogenase (quinone) (EC 1.6.5.10) is an enzyme that catalyzes the chemical reaction

In enzymology, a NADPH—hemoprotein reductase is an enzyme that catalyzes the chemical reaction
In enzymology, a nitrite reductase [NAD(P)H] (EC 1.7.1.4) is an enzyme that catalyzes the chemical reaction

NAD(P)H dehydrogenase [quinone] 1 is an enzyme that in humans is encoded by the NQO1 gene. This protein-coding gene is a member of the NAD(P)H dehydrogenase (quinone) family and encodes a 2-electron reductase (enzyme). This FAD-binding protein forms homodimers and performs two-electron reduction of quinones to hydroquinones and of other redox dyes. It has a preference for short-chain acceptor quinones, such as ubiquinone, benzoquinone, juglone and duroquinone. This gene has an important paralog NQO2. This protein is located in the cytosol.
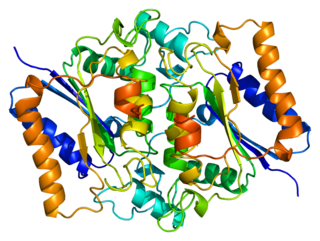
NAD(P)H dehydrogenase, quinone 2, also known as QR2, is a protein that in humans is encoded by the NQO2 gene. It is a phase II detoxification enzyme which can carry out two or four electron reductions of quinones. Its mechanism of reduction is through a ping-pong mechanism involving its FAD cofactor. Initially in a reductive phase NQO2 binds to reduced dihydronicotinamide riboside (NRH) electron donor, and mediates a hydride transfer from NRH to FAD. Then, in an oxidative phase, NQO2 binds to its quinone substrate and reduces the quinone to a dihydroquinone. Besides the two catalytic FAD, NQO2 also has two zinc ions. It is not clear whether the metal has a catalytic role. NQO2 is a paralog of NQO1
NQO2 is a homodimer. NQO2 can be inhibited by resveratrol. One of QR2's binding sites responds to 2-iodomelatonin, and has been referred to as MT3.

NADH:ubiquinone reductase (non-electrogenic) (EC 1.6.5.9, NDH-2, ubiquinone reductase, coenzyme Q reductase, dihydronicotinamide adenine dinucleotide-coenzyme Q reductase, DPNH-coenzyme Q reductase, DPNH-ubiquinone reductase, NADH-coenzyme Q oxidoreductase, NADH-coenzyme Q reductase, NADH-CoQ oxidoreductase, NADH-CoQ reductase) is an enzyme with systematic name NADH:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction:
Mycofactocin (MFT) is a family of small molecules derived from a peptide of the type known as RiPP (ribosomally synthesized and post-translationally modified peptides), naturally occurring in many types of Mycobacterium. It was discovered in a bioinformatics study in 2011. All mycofactocins share a precursor in the form of premycofactocin (PMFT); they differ by the cellulose tail added. Being redox active, both PMFT and MFT have an oxidized dione (mycofactocinone) form and a reduced diol (mycofactocinol) form, respectively termed PMFTH2 and MFTH2.










