
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects to float on a water surface without becoming even partly submerged.
In gas dynamics, Chaplygin's equation, named after Sergei Alekseevich Chaplygin (1902), is a partial differential equation useful in the study of transonic flow. It is
In physics, Washburn's equation describes capillary flow in a bundle of parallel cylindrical tubes; it is extended with some issues also to imbibition into porous materials. The equation is named after Edward Wight Washburn; also known as Lucas–Washburn equation, considering that Richard Lucas wrote a similar paper three years earlier, or the Bell-Cameron-Lucas-Washburn equation, considering J.M. Bell and F.K. Cameron's discovery of the form of the equation in 1906.

Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. There are two types of wetting: non-reactive wetting and reactive wetting.

The contact angle is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interface at their intersection. It quantifies the wettability of a solid surface by a liquid via the Young equation.

Cassie's law, or the Cassie equation, describes the effective contact angle θc for a liquid on a chemically heterogeneous surface, i.e. the surface of a composite material consisting of different chemistries, that is, non-uniform throughout. Contact angles are important as they quantify a surface's wettability, the nature of solid-fluid intermolecular interactions. Cassie's law is reserved for when a liquid completely covers both smooth and rough heterogeneous surfaces.
In fluid statics, capillary pressure is the pressure between two immiscible fluids in a thin tube, resulting from the interactions of forces between the fluids and solid walls of the tube. Capillary pressure can serve as both an opposing or driving force for fluid transport and is a significant property for research and industrial purposes. It is also observed in natural phenomena.
In physics, the Young–Laplace equation is an algebraic equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or wall tension, although use of the latter is only applicable if assuming that the wall is very thin. The Young–Laplace equation relates the pressure difference to the shape of the surface or wall and it is fundamentally important in the study of static capillary surfaces. It is a statement of normal stress balance for static fluids meeting at an interface, where the interface is treated as a surface :
A hydrogen-like atom (or hydrogenic atom) is any atom or ion with a single valence electron. These atoms are isoelectronic with hydrogen. Examples of hydrogen-like atoms include, but are not limited to, hydrogen itself, all alkali metals such as Rb and Cs, singly ionized alkaline earth metals such as Ca+ and Sr+ and other ions such as He+, Li2+, and Be3+ and isotopes of any of the above. A hydrogen-like atom includes a positively charged core consisting of the atomic nucleus and any core electrons as well as a single valence electron. Because helium is common in the universe, the spectroscopy of singly ionized helium is important in EUV astronomy, for example, of DO white dwarf stars.

The capillary length or capillary constant is a length scaling factor that relates gravity and surface tension. It is a fundamental physical property that governs the behavior of menisci, and is found when body forces (gravity) and surface forces are in equilibrium.
In fluid mechanics and mathematics, a capillary surface is a surface that represents the interface between two different fluids. As a consequence of being a surface, a capillary surface has no thickness in slight contrast with most real fluid interfaces.

In general relativity, a point mass deflects a light ray with impact parameter by an angle approximately equal to
Nuclear magnetic resonance (NMR) in porous materials covers the application of using NMR as a tool to study the structure of porous media and various processes occurring in them. This technique allows the determination of characteristics such as the porosity and pore size distribution, the permeability, the water saturation, the wettability, etc.
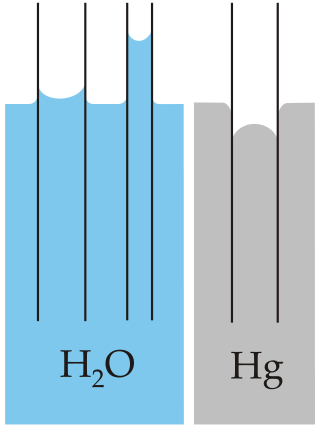
Jurin's law, or capillary rise, is the simplest analysis of capillary action—the induced motion of liquids in small channels—and states that the maximum height of a liquid in a capillary tube is inversely proportional to the tube's diameter. Capillary action is one of the most common fluid mechanical effects explored in the field of microfluidics. Jurin's law is named after James Jurin, who discovered it between 1718 and 1719. His quantitative law suggests that the maximum height of liquid in a capillary tube is inversely proportional to the tube's diameter. The difference in height between the surroundings of the tube and the inside, as well as the shape of the meniscus, are caused by capillary action. The mathematical expression of this law can be derived directly from hydrostatic principles and the Young–Laplace equation. Jurin's law allows the measurement of the surface tension of a liquid and can be used to derive the capillary length.
In optics, the Fraunhofer diffraction equation is used to model the diffraction of waves when the diffraction pattern is viewed at a long distance from the diffracting object, and also when it is viewed at the focal plane of an imaging lens.
In general relativity, the Weyl metrics are a class of static and axisymmetric solutions to Einstein's field equation. Three members in the renowned Kerr–Newman family solutions, namely the Schwarzschild, nonextremal Reissner–Nordström and extremal Reissner–Nordström metrics, can be identified as Weyl-type metrics.
In physics, the distorted Schwarzschild metric is the metric of a standard/isolated Schwarzschild spacetime exposed in external fields. In numerical simulation, the Schwarzschild metric can be distorted by almost arbitrary kinds of external energy–momentum distribution. However, in exact analysis, the mature method to distort the standard Schwarzschild metric is restricted to the framework of Weyl metrics.
Morris Muskat et al. developed the governing equations for multiphase flow in porous media as a generalisation of Darcy's equation for water flow in porous media. The porous media are usually sedimentary rocks such as clastic rocks or carbonate rocks.
In fluid mechanics, the thin-film equation is a partial differential equation that approximately predicts the time evolution of the thickness h of a liquid film that lies on a surface. The equation is derived via lubrication theory which is based on the assumption that the length-scales in the surface directions are significantly larger than in the direction normal to the surface. In the non-dimensional form of the Navier-Stokes equation the requirement is that terms of order ε2 and ε2Re are negligible, where ε ≪ 1 is the aspect ratio and Re is the Reynolds number. This significantly simplifies the governing equations. However, lubrication theory, as the name suggests, is typically derived for flow between two solid surfaces, hence the liquid forms a lubricating layer. The thin-film equation holds when there is a single free surface. With two free surfaces, the flow must be treated as a viscous sheet.
In fluid dynamics, Landau–Squire jet or Submerged Landau jet describes a round submerged jet issued from a point source of momentum into an infinite fluid medium of the same kind. This is an exact solution to the incompressible form of the Navier-Stokes equations, which was first discovered by Lev Landau in 1944 and later by Herbert Squire in 1951. The self-similar equation was in fact first derived by N. A. Slezkin in 1934, but never applied to the jet. Following Landau's work, V. I. Yatseyev obtained the general solution of the equation in 1950.




















