
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
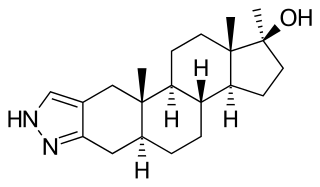
Stanozolol, sold under many brand names, is an androgen and anabolic steroid (AAS) medication derived from dihydrotestosterone (DHT). It is used to treat hereditary angioedema. It was developed by American pharmaceutical company Winthrop Laboratories in 1962, and has been approved by the U.S. Food and Drug Administration for human use, though it is no longer marketed in the USA. It is also used in veterinary medicine. Stanozolol has mostly been discontinued, and remains available in only a few countries. It is given by mouth in humans or by injection into muscle in animals.

Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.

Sterol is an organic compound with formula C
17H
28O, whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria. The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones.

Sex hormone-binding globulin (SHBG) or sex steroid-binding globulin (SSBG) is a glycoprotein that binds to androgens and estrogens. When produced by the Sertoli cells in the seminiferous tubules of the testis, it is called androgen-binding protein (ABP).

Metandienone, also known as methandienone or methandrostenolone and sold under the brand name Dianabol (D-Bol) among others, is an androgen and anabolic steroid (AAS) medication which is still quite often used because of its affordability and effectiveness for bulking cycles. It is also used non-medically for physique- and performance-enhancing purposes. It is often taken by mouth.
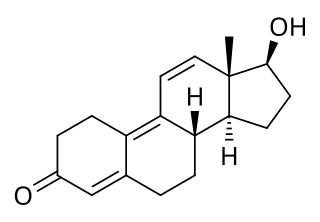
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.

Luis Ernesto Miramontes Cárdenas was a Mexican chemist known as the co-inventor of the progestin norethisterone used in one of the first three oral contraceptives.

Ethylestrenol, also known as ethyloestrenol or ethylnandrol and sold under the brand names Maxibolin and Orabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used in the past for a variety of indications such as to promote weight gain and to treat anemia and osteoporosis but has been discontinued for use in humans. It is still available for veterinary use in Australia and New Zealand however. It is taken by mouth.

Androstenol, also known as 5α-androst-16-en-3α-ol, is a 16-androstene class steroidal pheromone and neurosteroid in humans and other mammals, notably pigs. It possesses a characteristic musk-like odor.
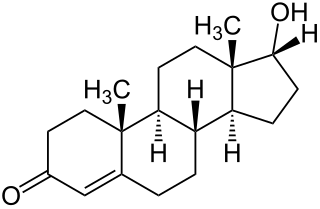
Epitestosterone, or isotestosterone, also known as 17α-testosterone or as androst-4-en-17α-ol-3-one, is an endogenous steroid and an epimer of the androgen sex hormone testosterone. It is a weak competitive antagonist of the androgen receptor (AR) and a potent 5α-reductase inhibitor.

Trilostane, sold under the brand names Modrenal and Vetoryl among others, is a medication which has been used in the treatment of Cushing's syndrome, Conn's syndrome, and postmenopausal breast cancer in humans. It was withdrawn for use in humans in the United States in the 1990s but was subsequently approved for use in veterinary medicine in the 2000s to treat Cushing's syndrome in dogs. It is taken by mouth.

Steroid 21-hydroxylase is an enzyme that hydroxylates steroids at the C21 position and is involved in biosynthesis of aldosterone and cortisol. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively, within metabolic pathways that ultimately lead to aldosterone and cortisol. Deficiency in the enzyme may cause congenital adrenal hyperplasia.

The Report to the Commissioner of Baseball of an Independent Investigation into the Illegal Use of Steroids and Other Performance Enhancing Substances by Players in Major League Baseball, informally known as the Mitchell Report, is the result of former Democratic United States Senator from Maine George J. Mitchell's 20-month investigation into the use of anabolic steroids and human growth hormone (HGH) in Major League Baseball (MLB). The 409-page report, released on December 13, 2007, covers the history of the use of illegal performance-enhancing substances by players and the effectiveness of the MLB Joint Drug Prevention and Treatment Program. The report also advances certain recommendations regarding the handling of past illegal drug use and future prevention practices. In addition, the report names 89 MLB players who are alleged to have used steroids or other performance-enhancing drugs.

Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are synthetic substances that mimic the effects of testosterone, the male sex hormone. They are used to increase muscle size, strength, and performance and are commonly associated with athletic performance enhancement and bodybuilding. Anabolic steroids are classified as Schedule III controlled substances in many countries due to their potential for abuse and adverse health effects.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.
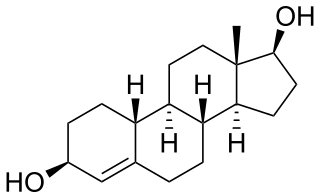
Bolandiol is an anabolic-androgenic steroid (AAS) that was never marketed. However, a dipropionate ester derivative, bolandiol dipropionate, has been marketed. Bolandiol and its dipropionate ester are unique among AASs in that they reportedly also possesses estrogenic and progestogenic activity.

Trenbolone hexahydrobenzylcarbonate, or trenbolone cyclohexylmethylcarbonate, sold under the brand names Parabolan and Hexabolan, is a synthetic, injected anabolic–androgenic steroid (AAS) of the nandrolone group and an androgen ester – specifically, the C17β hexahydrobenzylcarbonate (cyclohexylmethylcarbonate) ester of trenbolone – which was marketed in France for medical use in humans but has since been discontinued.
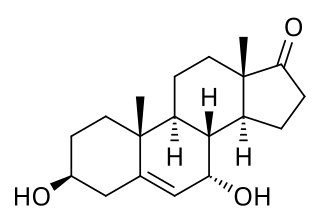
7α-Hydroxydehydroepiandrosterone, also known as 3β,7α-dihydroxyandrost-5-ene-17-one, is an endogenous, naturally occurring steroid and a major metabolite of dehydroepiandrosterone (DHEA) that is formed by CYP7B1 in tissues such as the prostate gland and by CYP3A4 in the liver. The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA. 7α-OH-DHEA has weak estrogenic activity, selectively activating the estrogen receptor ERβ. In addition, 7α-OH-DHEA may be responsible for the known antiglucocorticoid effects of DHEA.



















