
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

Alamethicin is a channel-forming peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib. This residue strongly induces formation of alpha-helical structure. The peptide sequence is:

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
The C-terminus is the end of an amino acid chain, terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus.
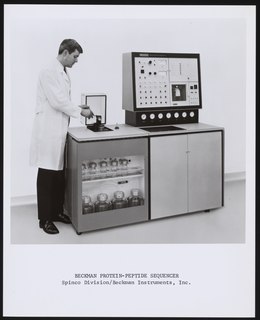
Protein sequencing is the practical process of determining the amino acid sequence of all or part of a protein or peptide. This may serve to identify the protein or characterize its post-translational modifications. Typically, partial sequencing of a protein provides sufficient information to identify it with reference to databases of protein sequences derived from the conceptual translation of genes.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.
Neurophysin I is a carrier protein with a size of 10 KDa and contains 90 to 97 amino acids. It is a cleavage product of preprooxyphysin. It is a neurohypophysial hormone that is transported in vesicles with oxytocin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with oxytocin and released with oxytocin, its biological action is unclear.
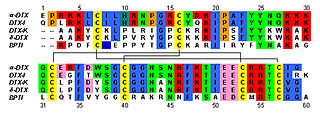
Dendrotoxins are a class of presynaptic neurotoxins produced by mamba snakes (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel proteins.
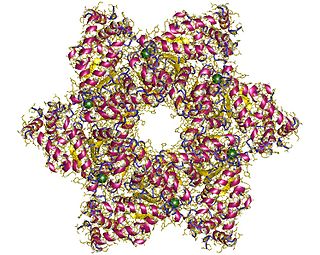
SV40 large T antigen is a hexamer protein that is a dominant-acting oncoprotein derived from the polyomavirus SV40. TAg is capable of inducing malignant transformation of a variety of cell types. The transforming activity of TAg is due in large part to its perturbation of the retinoblastoma (pRb) and p53 tumor suppressor proteins. In addition, TAg binds to several other cellular factors, including the transcriptional co-activators p300 and CBP, which may contribute to its transformation function.

Bovine pancreatic ribonuclease, also often referred to as bovine pancreatic ribonuclease A or simply RNase A, is a pancreatic ribonuclease enzyme that cleaves single-stranded RNA. Bovine pancreatic ribonuclease is one of the classic model systems of protein science. Two Nobel Prizes in Chemistry have been awarded in recognition of work on bovine pancreatic ribonuclease: in 1972, the Prize was awarded to Christian Anfinsen for his work on protein folding and to Stanford Moore and William Stein for their work on the relationship between the protein's structure and its chemical mechanism; in 1984, the Prize was awarded to Robert Bruce Merrifield for development of chemical synthesis of proteins.

Barnase (a portmanteau of "BActerial" "RiboNucleASE") is a bacterial protein that consists of 110 amino acids and has ribonuclease activity. It is synthesized and secreted by the bacterium Bacillus amyloliquefaciens, but is lethal to the cell when expressed without its inhibitor barstar. The inhibitor binds to and occludes the ribonuclease active site, preventing barnase from damaging the cell's RNA after it has been synthesized but before it has been secreted. The barnase/barstar complex is noted for its extraordinarily tight protein-protein binding, with an on-rate of 108s−1M−1.

Prolyl isomerase is an enzyme found in both prokaryotes and eukaryotes that interconverts the cis and trans isomers of peptide bonds with the amino acid proline. Proline has an unusually conformationally restrained peptide bond due to its cyclic structure with its side chain bonded to its secondary amine nitrogen. Most amino acids have a strong energetic preference for the trans peptide bond conformation due to steric hindrance, but proline's unusual structure stabilizes the cis form so that both isomers are populated under biologically relevant conditions. Proteins with prolyl isomerase activity include cyclophilin, FKBPs, and parvulin, although larger proteins can also contain prolyl isomerase domains.

Slotoxin is a peptide from Centruroides noxius Hoffmann scorpion venom. It belongs to the short scorpion toxin superfamily.

Neurophysin II is a carrier protein with a size of 19,687.3 Da and is made up of a dimer of two virtually identical chains of amino acids. Neurophysin II is a cleavage product of the AVP gene. It is a neurohypophysial hormone that is transported in vesicles with vasopressin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with vasopressin and released with vasopressin into the bloodstream, its biological action is unclear. Neurophysin II is also known as a stimulator of prolactin secretion.

Ribonuclease T1 (EC 3.1.27.3, guanyloribonuclease, Aspergillus oryzae ribonuclease, RNase N1, RNase N2, ribonuclease N3, ribonuclease U1, ribonuclease F1, ribonuclease Ch, ribonuclease PP1, ribonuclease SA, RNase F1, ribonuclease C2, binase, RNase Sa, guanyl-specific RNase, RNase G, RNase T1, ribonuclease guaninenucleotido-2'-transferase (cyclizing), ribonuclease N3, ribonuclease N1) is a fungal endonuclease that cleaves single-stranded RNA after guanine residues, i.e., on their 3' end; the most commonly studied form of this enzyme is the version found in the mold Aspergillus oryzae. Owing to its specificity for guanine, RNase T1 is often used to digest denatured RNA prior to sequencing. Similar to other ribonucleases such as barnase and RNase A, ribonuclease T1 has been popular for folding studies.
Orexin-A, also known as hypocretin-1, is a naturally occurring neuropeptide and orexin isoform. The orexinergic nucleus in the lateral hypothalamus is the primary orexin projection system in the brain.

Beefy meaty peptide, also known as delicious peptide and abbreviated as BMP, is an 8-amino acid long peptide that has been identified as giving a beefy flavour to foods in which it is present. It was isolated from beef soup by Yamasaki and Maekawa in 1978. Ongoing research since its discovery by Yamasaki and Maekawa has provided general support for the presence of its flavor-imparting properties. However, due to its high production cost, the peptide's potential for widespread application in the food industry has yet to be realized, prompting current research efforts to focus on finding a method of mass-production for the peptide.
A myc tag is a polypeptide protein tag derived from the c-myc gene product that can be added to a protein using recombinant DNA technology. It can be used for affinity chromatography, then used to separate recombinant, overexpressed protein from wild type protein expressed by the host organism. It can also be used in the isolation of protein complexes with multiple subunits.

Klaus H. Hofmann was an American biological chemist and medical researcher. The New York Times called Hofmann an "expert on synthesis of body compounds". His career was highlighted by synthesis of a prototype birth control pill, isolation and structural characterization of biotin, determination of the lysine specificity of the pancreatic protease trypsin, the first chemical synthesis of a fully biologically-active portion of the peptide hormone, and structure-function studies on ribonuclease (RNase).
1. R.T. Raines et al., The S-Tag Fusion System for Protein Purification. Methods Enzymol. 326, 362-367 (2000)















