
Interleukin 8 is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel–Palade bodies. In humans, the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms. In culture, a 72 amino acid peptide is the major form secreted by macrophages.

The S100 proteins are a family of low molecular-weight proteins found in vertebrates characterized by two calcium-binding sites that have helix-loop-helix ("EF-hand-type") conformation. At least 21 different S100 proteins are known. They are encoded by a family of genes whose symbols use the S100 prefix, for example, S100A1, S100A2, S100A3. They are also considered as damage-associated molecular pattern molecules (DAMPs), and knockdown of aryl hydrocarbon receptor downregulates the expression of S100 proteins in THP-1 cells.

Calgranulin is an S100 calcium-binding protein that is expressed in multiple cell types, including renal epithelial cells and neutrophils.
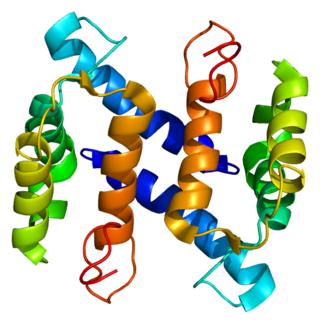
Protein S100-A4 (S100A4) is a protein that in humans is encoded by the S100A4 gene.

S100 calcium-binding protein A2 (S100A2) is a protein that in humans is encoded by the S100A2 gene and it is located on chromosome 1q21 with other S100 proteins.

S100 calcium-binding protein B (S100B) is a protein of the S100 protein family.

S100 calcium-binding protein A8 (S100A8) is a protein that in humans is encoded by the S100A8 gene. It is also known as calgranulin A.

S100 calcium-binding protein A9 (S100A9) also known as migration inhibitory factor-related protein 14 (MRP14) or calgranulin B is a protein that in humans is encoded by the S100A9 gene.

S100 calcium-binding protein A6 (S100A6) is a protein that in humans is encoded by the S100A6 gene.

S100 calcium-binding protein A11 (S100A11) is a protein that in humans is encoded by the S100A11 gene.
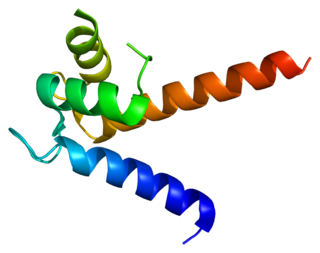
S100 calcium-binding protein P (S100P) is a protein that in humans is encoded by the S100P gene.

S100 calcium-binding protein A13 (S100A13) is a protein that in humans is encoded by the S100A13 gene.

S100 calcium-binding protein A3 (S100A3) is a protein that in humans is encoded by the S100A3 gene.
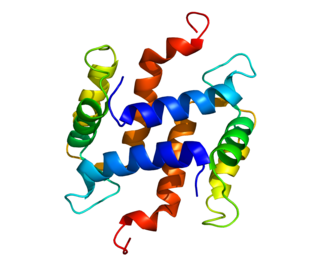
S100 calcium-binding protein A5 (S100A5) is a protein that is encoded by the S100A5 gene in humans.
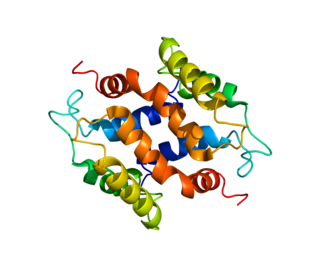
S100 calcium-binding protein A16 (S100A16) is a protein that in humans is encoded by the S100A16 gene.
Calprotectin is a complex of the mammalian proteins S100A8 and S100A9. Other names for calprotectin include MRP8-MRP14, calgranulin A and B, cystic fibrosis antigen, L1, 60BB antigen, and 27E10 antigen. The proteins exist as homodimers but preferentially exist as S100A8/A9 heterodimers or heterotetramers (calprotectin) with antimicrobial, proinflammatory and prothrombotic properties. In the presence of calcium, calprotectin is capable of sequestering the transition metals iron, manganese and zinc via chelation. This metal sequestration affords the complex antimicrobial properties. Calprotectin is the only known antimicrobial manganese sequestration protein complex. Calprotectin comprises as much as 60% of the soluble protein content of the cytosol of a neutrophil, and it is secreted by an unknown mechanism during inflammation. Faecal calprotectin has been used to detect intestinal inflammation and can serve as a biomarker for inflammatory bowel diseases. Blood-based calprotectin is used in diagnostics of multiple inflammatory diseases, including autoimmune diseases, like arthritis, and severe infections including sepsis.

Protein S100-A7A (S100A7A), also known as koebnerisin, is a protein that in humans is encoded by the S100A7A gene.

S100 calcium binding protein A14 (S100A14) is a protein that in humans is encoded by the S100A14 gene.
The S100 calcium-binding protein mS100a7a15 is the murine ortholog of human S100A7 (Psoriasin) and human S100A15 (Koebnerisin). mS100a7a15 is also known as S100a15, mS100a7 and mS100a7a and is encoded by the mS100a7a gene
The epidermal differentiation complex (EDC) is a gene complex comprising over fifty genes encoding proteins involved in the terminal differentiation and cornification of keratinocytes, the primary cell type of the epidermis. In humans, the complex is located on a 1.9 Mbp stretch within chromosome 1q21. The proteins encoded by EDC genes are closely related in terms of function, and evolutionarily they belong to three distinct gene families: the cornified envelope precursor family, the S100 protein family and the S100 fused type protein (SFTP) family.






















