| Influenza (Flu) |
|---|
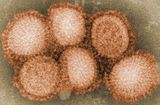 |
Seasonal influenza vaccine brands include Fluzone/Fluzone Quadrivalent [1] and Vaxigrip/VaxigripTetra, [2] Influvac and Optaflu.
| Influenza (Flu) |
|---|
 |
Seasonal influenza vaccine brands include Fluzone/Fluzone Quadrivalent [1] and Vaxigrip/VaxigripTetra, [2] Influvac and Optaflu.
CSL Limited obtain Novartis' flu vaccine unit in 2014, and transfer to CSL Subsidiary, bioCSL (Seqirus). [7] The following are list of bioCSL flu vaccine brands: [8]
Fluarix and Flulaval are flu vaccines brand of GlaxoSmithKline. [15]
Influvac is a subunit vaccine produced and marketed by Mylan. It contains inactivated purified surface fragments (subunits) from the three different strains of the influenza virus (A/H1N1, A/H3N2, and Influenza B virus) that are selected and distributed by the World Health Organization, on the basis of their latest recommendations. Previously, it was produced and marketed by Abbott Laboratories [16]
In February 2010, Abbott acquired the vaccines subunit from Solvay Pharmaceuticals included in its $6.2 billion purchase [17] and the subunit influenza vaccine — Influvac has been commercially available on the market since the early nineteen-eighties. [16] With the acquisition of Solvay, Abbott retained access to the Eastern European, Middle Eastern & Latin American markets. Approximately $850 million of sales revenue from vaccines was reported by Solvay Pharmaceuticals in 2009. [17]
In February 2015, Mylan Laboratories completed the deal with Abbott to purchase Abbott's generic drugs business in developed markets, which includes Influvac. [18] [19]
Optaflu is a cell culture derived influenza vaccine manufactured by Novartis. On April 27, 2007 Novartis received a positive opinion supporting European Union approval of Optaflu. It is the first influenza vaccine made in a mammalian cell line, rather than chicken eggs. [20] The plan was to manufacture the vaccine in Holly Springs, North Carolina. The United States government provided $500 million in construction costs and guaranteed vaccine purchases. [21]
Novartis' flu vaccine unit was sold to CSL Limited in 2014, and was placed under CSL subsidiary, bioCSL (Seqirus). [7] bioCSL as marketing authorization holder decided to discontinue the usage of Optaflu brand in 2017 due to commercial reasons [22]
Sanofi Pasteur produces the split-virus influenza vaccines Fluzone (especially in the United States [1] and Vaxigrip/VaxigripTetra (especially in Europe). [2] Sanofi also produces a recombinant influenza vaccine branded Flublok. [23]

Fluzone is a brand of influenza vaccine distributed by Sanofi Pasteur. It is a split-virus vaccine that is produced by chemical disruption of the influenza virus, making it incapable of causing influenza.[ citation needed ]
Fluzone is typically administered in a single dose by intramuscular injection; [24] an intradermal injection is also available. [25] It is presented as a 0.25 ml syringe for pediatric use, as a 0.5 ml syringe for adults and children, as a 0.5 ml vial for adults and children, and as a 5 ml vial for adults and children. [24] Fluzone must be refrigerated under temperatures from 2 to 8 °C (36 to 46 °F) and is inactivated by freezing. Fluzone was initially approved in 1980 by the FDA. [24]
The following adverse effects have been reported: [24]
A high-dose vaccine (Fluzone High-Dose) four times the strength of standard flu vaccine was approved by the FDA in 2009. [26] [27] [28] This vaccine is intended for people 65 and over, who typically have weakened immune response due to normal aging. The vaccine produces a greater immune response than standard vaccine. According to the CDC, [1] "a study published in the New England Journal of Medicine [29] [in August, 2014] indicated that the high-dose vaccine was 24.2% more effective in preventing flu in adults 65 years of age and older relative to a standard-dose vaccine." The CDC recommends the high-dose vaccine for people 65 and over but expresses no preference between it and standard vaccine. Further studies were underway as of 2014 [update] .[ citation needed ]
The split-virion influenza vaccines made by Sanofi Pasteur in Europe are called Vaxigrip and VaxigripTetra. [2] [30] Vaxigrip provides immune responses to three influenza strains and VaxigripTetra adds another B strain. VaxigripTetra was approved in Europe in 2016 except for infants younger than three years old. [2]
Recombinant influenza vaccines are produced using recombinant virus technology. This method does not require an egg-grown vaccine virus and does not use chicken eggs in the production process. Sanofi's recombinant influenza vaccine is called Flublok. [31] [32] [23]

The DPT vaccine or DTP vaccine is a class of combination vaccines against three infectious diseases in humans: diphtheria, pertussis, and tetanus. The vaccine components include diphtheria and tetanus toxoids and either killed whole cells of the bacterium that causes pertussis or pertussis antigens. The whole cells or antigens will be depicted as either "DTwP" or "DTaP", where the lower-case "w" indicates whole-cell inactivated pertussis and the lower-case "a" indicates pertussis antigens.

Influenza vaccines, also known as flu shots or flu jabs, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high quality research. Vaccinating children may protect those around them.
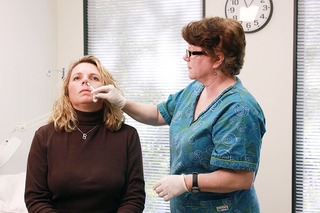
Live attenuated influenza vaccine (LAIV) is a type of influenza vaccine in the form of a nasal spray that is recommended for the prevention of influenza. In June 2016, the Centers for Disease Control and Prevention (CDC) stopped recommending the use of LAIV as its effectiveness had appeared to have decreased between 2013 and 2016, but this recommendation was reversed in February 2018, for the 2018-2019 influenza season.

CSL Limited is a global specialty biotechnology company that researches, develops, manufactures, and markets products to treat and prevent serious human medical conditions. CSL's product areas include blood plasma derivatives, vaccines, antivenom, and cell culture reagents used in various medical and genetic research and manufacturing applications.

Influenza A virus subtype H3N2 (A/H3N2) is a subtype of viruses that causes influenza (flu). H3N2 viruses can infect birds and mammals. In birds, humans, and pigs, the virus has mutated into many strains. In years in which H3N2 is the predominant strain, there are more hospitalizations.

Influenza research involves investigating molecular virology, pathogenesis, host immune responses, genomics, and epidemiology regarding influenza. The main goal of research is to develop influenza countermeasures such as vaccines, therapies and diagnostic tools.
The MMRV vaccine combines the attenuated virus MMR vaccine with the addition of the chickenpox vaccine or varicella vaccine. The MMRV vaccine is typically given to children between one and two years of age.

H5N1 clinical trials are clinical trials concerning H5N1 vaccines, which are intended to provide immunization to influenza A virus subtype H5N1. They are intended to discover pharmacological effects and identify any adverse reactions the vaccines may achieve in humans.

Twinrix is a vaccine against hepatitis A and hepatitis B, manufactured by GlaxoSmithKline Biologicals. The full generic name is hepatitis A inactivated & hepatitis B (recombinant) vaccine. Twinrix is administered over three doses.
Zoster vaccines are two vaccines that have been shown to reduce the rates of herpes zoster. One type, Zostavax, is essentially a larger-than-normal dose of the chickenpox vaccine, as both shingles and chickenpox are caused by the same virus, the varicella zoster virus (VZV). A recombinant version, Shingrix, was approved in the United States in 2017.
Hepatitis A vaccine is a vaccine that prevents hepatitis A. It is effective in around 95% of cases and lasts for at least fifteen years and possibly a person's entire life. If given, two doses are recommended beginning after the age of one. It is given by injection into a muscle.

Pandemrix is an influenza vaccine for influenza pandemics, such as the 2009 flu pandemic. The vaccine was developed by GlaxoSmithKline (GSK) and patented in September 2006.
Meningococcal vaccine refers to any of the vaccines used to prevent infection by Neisseria meningitidis. Different versions are effective against some or all of the following types of meningococcus: A, B, C, W-135, and Y. The vaccines are between 85 and 100% effective for at least two years. They result in a decrease in meningitis and sepsis among populations where they are widely used. They are given either by injection into a muscle or just under the skin.

The 2009 flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed vaccine was injected, while the live vaccine was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009.
AS03 is the trade name for a squalene-based immunologic adjuvant used in various vaccine products by GlaxoSmithKline (GSK). It is used, for example, in GSK's A/H1N1 pandemic flu vaccine Pandemrix. It is also in Arepannix and the new Q-pan for H5N1 influenza.

A H5N1 vaccine is an influenza vaccine intended to provide immunization to influenza A virus subtype H5N1.
Cell-based vaccines are developed from mammalian cell lines rather than the more common method which uses the cells in embryonic chicken eggs to develop the antigens. The potential use of cell culture techniques in developing viral vaccines has been widely investigated in recent years as a complementary and alternative platform to the current egg-based strategies.
DTaP-IPV-HepB vaccine is a combination vaccine whose generic name is diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B (recombinant) and inactivated poliovirus vaccine or DTaP-IPV-Hep B. It protects against the infectious diseases diphtheria, tetanus, pertussis, poliomyelitis, and hepatitis B.
A hexavalent vaccine, or 6-in-1 vaccine, is a combination vaccine with six individual vaccines conjugated into one, intended to protect people from multiple diseases. The principal example is a pediatric vaccine, used in more than 90 countries around the world including in Europe, Canada, Australia, and New Zealand that protects against diphtheria, tetanus, pertussis, poliomyelitis, haemophilus B, and hepatitis B.

A nasal vaccine is a vaccine administered to a person via the nose and does not require a needle. It induces immunity through the inner surface of the nose, a surface that naturally comes in contact with many airborne microbes.
But Novartis is building a cell culture flu vaccine factory in Holly Springs, N.C., which might be ready for use in 2010 or 2011. The federal government is providing nearly $500 million in construction costs and guaranteed vaccine purchases.