
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver and are a major component of human skin oils.
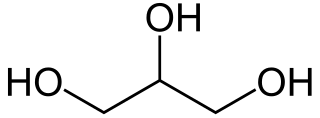
Glycerol is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
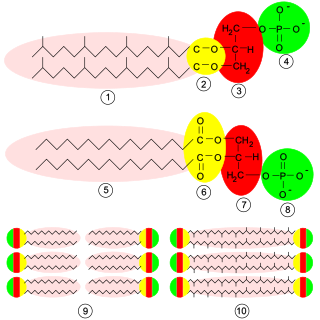
Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes in eukaryotic cells. They are a type of lipid, of which its composition affects membrane structure and properties. Two major classes are known: those for bacteria and eukaryotes and a separate family for archaea.
Platelet-activating factor, also known as PAF, PAF-acether or AGEPC (acetyl-glyceryl-ether-phosphorylcholine), is a potent phospholipid activator and mediator of many leukocyte functions, platelet aggregation and degranulation, inflammation, and anaphylaxis. It is also involved in changes to vascular permeability, the oxidative burst, chemotaxis of leukocytes, as well as augmentation of arachidonic acid metabolism in phagocytes.

Shark liver oil is an oil obtained from the livers of sharks. It has been used for centuries as a folk remedy to promote the healing of wounds and as a remedy for respiratory tract and digestive system problems. It is still promoted as a dietary supplement, and additional claims have been made that it can treat other maladies such as cancer, HIV, radiation sickness, swine flu, and the common cold. To date, none of these claims has been medically validated and shark liver oil (alone) is not a medication prescribed or utilized by American physicians. However, it is a component of some moisturizing skin lotions and hemorrhoid medications.

In biochemistry, an ether lipid refers to any lipid in which the lipid "tail" group is attached to the glycerol backbone via an ether bond at any position. In contrast, conventional glycerophospholipids and triglycerides are triesters. Structural types include:
Cetyl alcohol, also known as hexadecan-1-ol and palmityl alcohol, is a C-16 fatty alcohol with the formula CH3(CH2)15OH. At room temperature, cetyl alcohol takes the form of a waxy white solid or flakes. The name cetyl refers to whale oil (cetacea oil, from Latin: cetus, lit. 'whale', from Ancient Greek: κῆτος, romanized: kētos, lit. 'huge fish') from which it was first isolated.

In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.

Monoglycerides are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol.

The gulper shark is a long and slender dogfish usually about three feet in length generally found in deep, murky waters all around the world. It is a light grayish brown, paler ventrally, with a long snout and large greenish eyes. This deep water shark has two dorsal fins with long, grooved spines and the second dorsal fin smaller than the first. Its upper teeth are blade-like and lower have finely serrated edges. This tertiary consumer feeds on mainly fish such as bony fish, but also cephalopods such as squid and other invertebrates like crustaceans. The gulper shark is currently an endangered species mainly because of exploitation by humans and their abnormally long gestation period and low fecundity, preventing their population from recovering. Because of the depth of their habitat, they are considered little to no threat to humans.

The Portuguese dogfish or Portuguese shark, is a species of sleeper shark of the family Somniosidae. This globally distributed species has been reported down to a depth of 3,675 m (12,057 ft), making it the deepest-living shark known. It inhabits lower continental slopes and abyssal plains, usually staying near the bottom. Stocky and dark brown in color, the Portuguese dogfish can be distinguished from similar-looking species by the small spines in front of its dorsal fins. Its dermal denticles are also unusual, resembling the scales of a bony fish. This species typically reaches 0.9–1 m (3.0–3.3 ft) in length; sharks in the Mediterranean Sea are much smaller and have distinct depth and food preferences.

Glycidol is an organic compound with the formula HOCH2CHOCH2. The molecule contains both epoxide and alcohol functional groups. Being simple to make and bifunctional, it has a variety of industrial uses. The compound is a colorless, slightly viscous liquid that is slightly unstable and is not often encountered in pure form.
Alkylglycerol monooxygenase (AGMO) is an enzyme that catalyzes the hydroxylation of alkylglycerols, a specific subclass of ether lipids. This enzyme was first described in 1964 as a pteridine-dependent ether lipid cleaving enzyme. In 2010 finally, the gene coding for alkylglycerol monooxygenase was discovered as transmembrane protein 195 (TMEM195) on chromosome 7. In analogy to the enzymes phenylalanine hydroxylase, tyrosine hydroxylase, tryptophan hydroxylase and nitric oxide synthase, alkylglycerol monooxygenase critically depends on the cofactor tetrahydrobiopterin and iron.

2-Arachidonyl glyceryl ether is a putative endocannabinoid discovered by Lumír Hanuš and colleagues at the Hebrew University of Jerusalem, Israel. It is an ether formed from the alcohol analog of arachidonic acid and glycerol. Its isolation from porcine brain and its structural elucidation and synthesis were described in 2001.

sn-Glycerol 1-phosphate is the conjugate base of a phosphoric ester of glycerol. It is a component of ether lipids, which are common for archaea.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.
Batyl alcohol is an organic compound with the formula HOCH2CH(OH)CH2OC18H37. It is a colorless solid. Batyl alcohol is a monoether formed by condensation of stearyl alcohol with one of the two primary alcohol sites of glycerol. Together with S-selachyl alcohol and S-chimyl alcohol, S-batyl alcohol is a component of some lipid membranes.

Chimyl alcohol is an organic compound with the formula HOCH2CH(OH)CH2OC16H33. It is a colorless solid. Chimyl alcohol is a monoether formed by condensation of cetyl alcohol with one of the two primary alcohol sites of glycerol. Together with S-selachyl alcohol and S-batyl alcohol, S-chimyl alcohol is a component of some lipid membranes. It is found in the liver of the shark Centrophorus squamosus. The name chimyl is derived from a classification of ratfish, order Chimaeriformes. Like other glyceryl ethers, those derived from chimyl alcohol are not saponifiable.















