
Dehydroalanine is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As an amino acid residue, it is unusual because it has an unsaturated backbone.
Dehydratases are a group of lyase enzymes that form double and triple bonds in a substrate through the removal of water. They can be found in many places including the mitochondria, peroxisome and cytosol. There are more than 150 different dehydratase enzymes that are classified into four groups. Dehydratases can act on hydroxyacyl-CoA with or without cofactors, and some have a metal and non-metal cluster act as their active site.

The enzyme argininosuccinate lyase (EC 4.3.2.1, ASL, argininosuccinase; systematic name 2-(N ω-L-arginino)succinate arginine-lyase (fumarate-forming)) catalyzes the reversible breakdown of argininosuccinate:

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:

The transsulfuration pathway is a metabolic pathway involving the interconversion of cysteine and homocysteine through the intermediate cystathionine. Two transsulfurylation pathways are known: the forward and the reverse.
The enzyme carbamoyl-serine ammonia-lyase (EC 4.3.1.13) catalyzes the chemical reaction
The enzyme diaminopropionate ammonia-lyase (EC 4.3.1.15) catalyzes the chemical reaction
The enzyme D-serine ammonia-lyase (EC 4.3.1.18), with systematic name D-serine ammonia-lyase (pyruvate-forming), catalyzes the chemical reaction
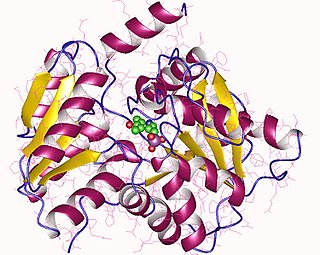
The enzyme L-serine ammonia-lyase (EC 4.3.1.17) catalyzes the chemical reaction
In enzymology, a S-alkylcysteine lyase is an enzyme that catalyzes the chemical reaction
The enzyme (2R)-sulfolactate sulfo-lyase catalyzes the reaction

Threonine ammonia-lyase (EC 4.3.1.19, systematic name L-threonine ammonia-lyase (2-oxobutanoate-forming), also commonly referred to as threonine deaminase or threonine dehydratase, is an enzyme responsible for catalyzing the conversion of L-threonine into α-ketobutyrate and ammonia:

The enzyme anthranilate synthase catalyzes the chemical reaction
The enzyme phosphatidylserine decarboxylase (EC 4.1.1.65) catalyzes the chemical reaction
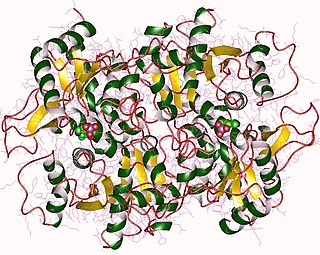
The enzyme threonine synthase (EC 4.2.3.1) catalyzes the chemical reaction
In enzymology, a cysteine synthase is an enzyme that catalyzes the chemical reaction

In molecular biology, the Cys/Met metabolism PLP-dependent enzyme family is a family of proteins including enzymes involved in cysteine and methionine metabolism which use PLP (pyridoxal-5'-phosphate) as a cofactor.











