
Brassicaceae or Cruciferae is a medium-sized and economically important family of flowering plants commonly known as the mustards, the crucifers, or the cabbage family. Most are herbaceous plants, while some are shrubs. The leaves are simple, lack stipules, and appear alternately on stems or in rosettes. The inflorescences are terminal and lack bracts. The flowers have four free sepals, four free alternating petals, two shorter free stamens and four longer free stamens. The fruit has seeds in rows, divided by a thin wall.

Horseradish is a perennial plant of the family Brassicaceae. It is a root vegetable, cultivated and used worldwide as a spice and as a condiment. The species is probably native to Southeastern Europe and Western Asia.

Brassica is a genus of plants in the cabbage and mustard family (Brassicaceae). The members of the genus are informally known as cruciferous vegetables, cabbages, mustard plants, or simply brassicas. Crops from this genus are sometimes called cole crops—derived from the Latin caulis, denoting the stem or stalk of a plant.

In organic chemistry, isothiocyanate is a functional group as found in compounds with the formula R−N=C=S. Isothiocyanates are the more common isomers of thiocyanates, which have the formula R−S−C≡N.

Mustard oil can mean either the pressed oil used for cooking, or a pungent essential oil also known as volatile oil of mustard. The essential oil results from grinding mustard seed, mixing the grounds with water, and isolating the resulting volatile oil by distillation. It can also be produced by dry distillation of the seed. Pressed mustard oil is used as cooking oil in some cultures, but sale is restricted in some countries due to high levels of erucic acid. Varieties of mustard seed low in erucic acid have been cultivated.

Allyl isothiocyanate (AITC) is a naturally occurring unsaturated isothiocyanate. The colorless oil is responsible for the pungent taste of cruciferous vegetables such as mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.

Brassica juncea, commonly brown mustard, Chinese mustard, Indian mustard, Korean green mustard, leaf mustard, Oriental mustard and vegetable mustard, is a species of mustard plant.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.

White mustard is an annual plant of the family Brassicaceae. It is sometimes also referred to as Brassica alba or B. hirta. Grown for its seeds, it is used to make the condiment mustard, as a fodder crop, or as a green manure. It is now widespread worldwide, although it probably originated in the Mediterranean region.
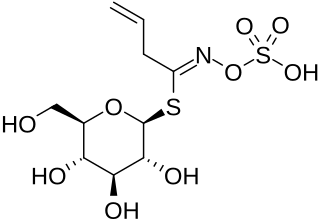
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard. Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil, which is responsible for the pungent taste of mustard and horseradish. Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.

Cruciferous vegetables are vegetables of the family Brassicaceae with many genera, species, and cultivars being raised for food production such as cauliflower, cabbage, kale, garden cress, bok choy, broccoli, Brussels sprouts, mustard plant and similar green leaf vegetables. The family takes its alternative name from the shape of their flowers, whose four petals resemble a cross.

Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion, which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue or in intact plants.

Rhamphospermum arvense, the charlock mustard, field mustard, wild mustard, or just charlock, is an annual or winter annual plant in the family Brassicaceae. It is found in the fields of North Africa, Asia, Europe, and some other areas where it has been transported and naturalized. Pieris rapae, the small white butterfly, and Pieris napi, the green veined white butterfly, are significant consumers of charlock during their larval stages.

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.

Gluconasturtiin or phenethyl glucosinolate is one of the most widely distributed glucosinolates in the cruciferous vegetables, mainly in the roots, and is probably one of the plant compounds responsible for the natural pest-inhibiting properties of growing crucifers, such as cabbage, mustard or rape, in rotation with other crops. This effect of gluconasturtiin is due to its degradation by the plant enzyme myrosinase into phenethyl isothiocyanate, which is toxic to many organisms.
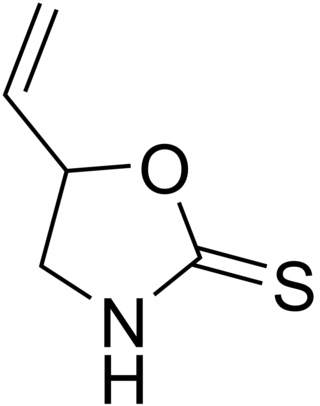
Goitrin is an organosulfur compound classified as a derivative of oxazolidine and as a cyclic thiocarbamate. It reduces the production of thyroid hormones such as thyroxine. It is found in cruciferous vegetables such as cabbage, brussels sprouts and rapeseed oil, and is formed by the hydrolysis of a glucosinolate: progoitrin or 2-hydroxy-3-butenyl glucosinolate. The unstable isothiocyanate derived from the latter glucosinolate spontaneously cyclizes to goitrin, because the hydroxy group is situated in proximity to the isothiocyanate group. Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate. Plants containing this specific glucosinolate have goitrogenic potential due to the goitrin and thiocyanate they contain. However, they do not seem to alter thyroid function in humans at realistic amounts in the diet.

Mustard is a condiment made from the seeds of a mustard plant.
Antifeedants are organic compounds produced by plants to repel herbivores through distaste or toxicity. These chemical compounds are typically classified as secondary metabolites in that they are not essential for the metabolism of the plant, but instead confer longevity. Antifeedants exhibit a wide range of activities and chemical structures as biopesticides. Examples include rosin, which inhibits attack on trees, and many alkaloids, which are highly toxic to specific insect species, such as quassinoids against the diamondback moth. Samadera indica also has quassinoids used for insect antifeedant uses.

4-Hydroxyphenylacetonitrile is a naturally occurring nitrile.

4-Hydroxybenzyl isothiocyanate is a naturally occurring isothiocyanate. It is formed as a degradation product of sinalbin from white mustard and is responsible for the pungent taste of mustard seeds.




















