| |
| Names | |
|---|---|
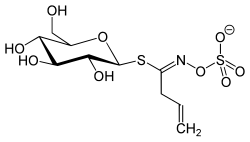
| IUPAC name (Z)-N-[1-(β-D-glucopyranosylsulfanyl)but-3-en-1-ylidene]hydroxylamine-O-sulfonic acid | |
| Systematic IUPAC name (Z)-N-(1-{{#parsoidfragment:0}}{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}but-3-en-1-ylidene)hydroxylamine-O-sulfonic acid | |
| Other names Allyl glucosinolate; 2-Propenyl glucosinolate; (1Z)-N-(Sulfooxy)but-3-enimidoyl 1-thio-β-D-glucopyranoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider | |
PubChem CID | |
| UNII |
|
| |
| |
| Properties | |
| C10H17NO9S2 | |
| Molar mass | 359.36 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard ( Brassica nigra ). Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil (allyl isothiocyanate), which is responsible for the pungent taste of mustard and horseradish. [1] Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.