
Radiation therapy or radiotherapy is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist.

A brain tumor occurs when a group of cells within the brain turn cancerous and grow out of control, creating a mass. There are two main types of tumors: malignant (cancerous) tumors and benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with vision, vomiting and mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness.

Colorectal cancer (CRC), also known as bowel cancer, colon cancer, or rectal cancer, is the development of cancer from the colon or rectum. Signs and symptoms may include blood in the stool, a change in bowel movements, weight loss, abdominal pain and fatigue. Most colorectal cancers are due to lifestyle factors and genetic disorders. Risk factors include diet, obesity, smoking, and lack of physical activity. Dietary factors that increase the risk include red meat, processed meat, and alcohol. Another risk factor is inflammatory bowel disease, which includes Crohn's disease and ulcerative colitis. Some of the inherited genetic disorders that can cause colorectal cancer include familial adenomatous polyposis and hereditary non-polyposis colon cancer; however, these represent less than 5% of cases. It typically starts as a benign tumor, often in the form of a polyp, which over time becomes cancerous.
Radionuclide therapy uses radioactive substances called radiopharmaceuticals to treat medical conditions, particularly cancer. These are introduced into the body by various means and localise to specific locations, organs or tissues depending on their properties and administration routes. This includes anything from a simple compound such as sodium iodide that locates to the thyroid via trapping the iodide ion, to complex biopharmaceuticals such as recombinant antibodies which are attached to radionuclides and seek out specific antigens on cell surfaces.

Brachytherapy is a form of radiation therapy where a sealed radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Greek word βραχύς, brachys, meaning "short-distance" or "short". Brachytherapy is commonly used as an effective treatment for cervical, prostate, breast, esophageal and skin cancer and can also be used to treat tumours in many other body sites. Treatment results have demonstrated that the cancer-cure rates of brachytherapy are either comparable to surgery and external beam radiotherapy (EBRT) or are improved when used in combination with these techniques. Brachytherapy can be used alone or in combination with other therapies such as surgery, EBRT and chemotherapy.
Transcatheter arterial chemoembolization (TACE) is a minimally invasive procedure performed in interventional radiology to restrict a tumor's blood supply. Small embolic particles coated with chemotherapeutic drugs are injected selectively through a catheter into an artery directly supplying the tumor. These particles both block the blood supply and induce cytotoxicity, attacking the tumor in several ways.

Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.

Neuroendocrine tumors (NETs) are neoplasms that arise from cells of the endocrine (hormonal) and nervous systems. They most commonly occur in the intestine, where they are often called carcinoid tumors, but they are also found in the pancreas, lung, and the rest of the body.
Technetium (99mTc) arcitumomab was a drug used for the diagnostic imaging of colorectal cancers, marketed by Immunomedics. It consisted of the Fab' fragment of a monoclonal antibody and a radionuclide, technetium-99m.
TheraSphere is a radiotherapy treatment for hepatocellular carcinoma (HCC) that consists of millions of microscopic, radioactive glass microspheres being infused into the arteries that feed liver tumors. These microspheres then embolize, lodging themselves in the liver's capillaries and bathing the malignancy in high levels of yttrium-90 radiation. It is currently approved as a Humanitarian Device, meaning effectiveness has not been proven, for patients as a neoadjuvant to surgery or transplantation by the U.S. Food and Drug Administration and is being used at a number of clinical centers in the United States.
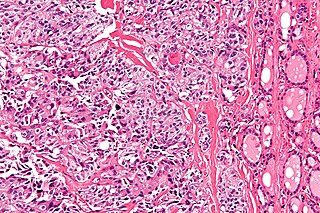
Medullary thyroid cancer is a form of thyroid carcinoma which originates from the parafollicular cells, which produce the hormone calcitonin. Medullary tumors are the third most common of all thyroid cancers and together make up about 3% of all thyroid cancer cases. MTC was first characterized in 1959.
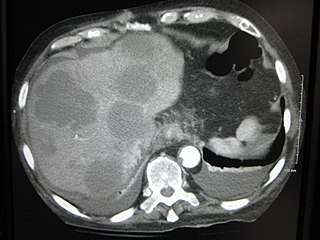
A liver metastasis is a malignant tumor in the liver that has spread from another organ that is affected by cancer. The liver is a common site for metastatic disease because of its rich, dual blood supply. Metastatic tumors in the liver are 20 times more common than primary liver tumors (tumors that originate in the liver(. In 50% of all cases the primary tumor is of the gastrointestinal tract; other common sites include the breast, ovaries, bronchus and kidney. Patients with colorectal cancer may also develop liver metastases.

Selective internal radiation therapy (SIRT), also known as transarterial radioembolization (TARE), radioembolization or intra-arterial microbrachytherapy is a form of radionuclide therapy used in interventional radiology to treat cancer. It is generally for selected patients with surgically unresectable cancers, especially hepatocellular carcinoma or metastasis to the liver. The treatment involves injecting tiny microspheres of radioactive material into the arteries that supply the tumor, where the spheres lodge in the small vessels of the tumor. Because this treatment combines radiotherapy with embolization, it is also called radioembolization. The chemotherapeutic analogue is called chemoembolization, of which transcatheter arterial chemoembolization (TACE) is the usual form.
In oncology, metastasectomy is the surgical removal of metastases, which are secondary cancerous growths that have spread from cancer originating in another organ in the body.
SIR-Spheres microspheres are used to treat patients with unresectable liver cancer. These are mostly patients with hepatocellular carcinoma (HCC), metastatic colorectal cancer (mCRC), or metastatic neuroendocrine tumours (mNET).
Yttrium-90 is a radioactive isotope of yttrium. Yttrium-90 has found a wide range of uses in radiation therapy to treat some forms of cancer. Along with other isotopes of yttrium, it is sometimes called radioyttrium.

A brain metastasis is a cancer that has metastasized (spread) to the brain from another location in the body and is therefore considered a secondary brain tumor. The metastasis typically shares a cancer cell type with the original site of the cancer. Metastasis is the most common cause of brain cancer, as primary tumors that originate in the brain are less common. The most common sites of primary cancer which metastasize to the brain are lung, breast, colon, kidney, and skin cancer. Brain metastases can occur months or even years after the original or primary cancer is treated. Brain metastases have a poor prognosis for cure, but modern treatments allow patients to live months and sometimes years after the diagnosis.

Plus Therapeutics, Inc. is a clinical-stage pharmaceutical company developing innovative, targeted radiotherapeutics for adults and children with rare and difficult-to-treat cancers. The company is headquartered in Austin, Texas, United States.
Interventional oncology is a subspecialty field of interventional radiology that deals with the diagnosis and treatment of cancer and cancer-related problems using targeted minimally invasive procedures performed under image guidance. Interventional oncology has developed to a separate pillar of modern oncology and it employs X-ray, ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) to help guide miniaturized instruments to allow targeted and precise treatment of solid tumours located in various organs of the human body, including but not limited to the liver, kidneys, lungs, and bones. Interventional oncology treatments are routinely carried out by interventional radiologists in appropriate settings and facilities.
Radiation lobectomy is a form of radiation therapy used in interventional radiology to treat liver cancer. It is performed in patients that would be surgical candidates for resection, but cannot undergo surgery due to insufficient remaining liver tissue. It consists of injecting small radioactive beads loaded with yttrium-90 into the hepatic artery feeding the hepatic lobe in which the tumor is located. This is done with the intent of inducing growth in the contralateral hepatic lobe, not dissimilarly from portal vein embolization (PVE).











