
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fiber.

Violet is the color of light at the short wavelength end of the visible spectrum, between blue and invisible ultraviolet. It is one of the seven colors that Isaac Newton labeled when dividing the spectrum of visible light in 1672. Violet light has a wavelength between approximately 380 and 435 nanometers. The color's name is derived from the violet flower.

Food coloring, or color additive, is any dye, pigment, or substance that imparts color when it is added to food or drink. They come in many forms consisting of liquids, powders, gels, and pastes. Food coloring is used in both commercial food production and domestic cooking. Food colorants are also used in a variety of non-food applications, including cosmetics, pharmaceuticals, home craft projects, and medical devices.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
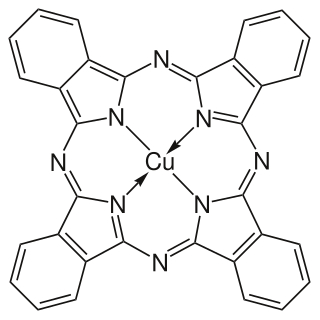
Copper phthalocyanine (CuPc), also called phthalocyanine blue, phthalo blue and many other names, is a bright, crystalline, synthetic blue pigment from the group of phthalocyanine dyes. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalis and acids. It has the appearance of a blue powder, insoluble in most solvents including water.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a different color when cool enough to drink, or kettles which change when water is at or near boiling point. Thermochromism is one of several types of chromism.
Vat dyes are a class of dyes that are classified as such because of the method by which they are applied. Vat dyeing is a process that refers to dyeing that takes place in a bucket or vat. The original vat dye is indigo, once obtained only from plants but now often produced synthetically.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Colored smoke is a kind of smoke created by an aerosol of small particles of a suitable pigment or dye.

Fuel dyes are dyes added to fuels, as in some countries it is required by law to dye a low-tax fuel to deter its use in applications intended for higher-taxed ones. Untaxed fuels are referred to as "dyed", while taxed ones are called "clear" or "white".

Victoria blue BO, also known as C.I. Basic Blue 7 and C.I. 42595, is a chloride salt of a dye with the chemical formula [C33H40N3]Cl. It has the appearance of a reddish blue powder. Victoria Blue BO base, also known as Solvent Blue 5 and C.I. 42595:1, is the hydroxide derivative of the same cation. Its chemical formula is [C33H4oN3]OH. Victoria blues are members of the triarylmethane dyes, but unlike most such dyes, the Victoria blues have a naphthylamine group.
A solvent dye is a dye soluble in organic solvents. It is usually used as a solution in an organic solvent.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.
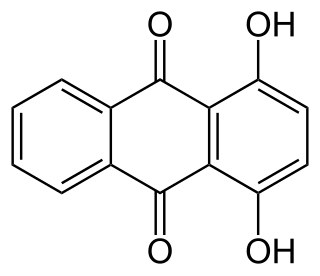
1,4-Dihydroxyanthraquinone, also called quinizarin or Solvent Orange 86, is an organic compound derived from anthroquinone. Quinizarin is an orange or red-brown crystalline powder. It is formally derived from anthraquinone by replacement of two hydrogen atoms by hydroxyl (OH) groups. It is one of ten dihydroxyanthraquinone isomers and occurs in small amounts in the root of the madder plant, Rubia tinctorum.

Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.
Methine dyes are dyes whose chromophoric system consists of conjugated double bonds (polyenes) flanked by two end groups: an electron acceptor A and an electron donor D.
Structural of methine dyes
The Bohn–Schmidt reaction, a named reaction in chemistry, introduces a hydroxy group at an anthraquinone system. The anthraquinone must already have at least one hydroxy group. The reaction was first described in 1889 by René Bohn (1862–1922) and in 1891 by Robert Emanuel Schmidt (1864–1938), two German industrial chemists.














