In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as monochloramine (NClH2).

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. Its main use is in the manufacture of precursors to polyurethane and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds.

Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

Azo compounds are compounds bearing the functional group diazenyl R−N=N−R′, in which R and R′ can be either aryl or alkyl.

Methyl yellow, or C.I. 11020, is an organic compound with the formula C6H5N2C6H4N(CH3)2. It is an azo dye derived from dimethylaniline. It is a yellow solid. According to X-ray crystallography, the C14N3 core of the molecule is planar.

Sudan I, is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.
Acid dyes are anionic, soluble in water and are essentially applied from acidic bath. These dyes possess acidic groups, such as SO3H and COOH and are applied on wool, silk and nylon when ionic bond is established between protonized –NH2 group of fibre and acid group of dye. Overall wash fastness is poor although light fastness is quite good. As dye and fibre contain opposite electrical nature, strike rate and uptake of acid dye on these fibres is faster; electrolyte at higher concentration is added to retard dye uptake and to form levelled shades. Acid generates cation on fibre and temperature helps to substitute negative part of acid with anionic dye molecules.

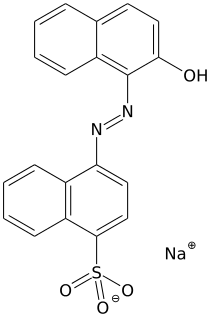
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl. They are a commercially important family of azo compounds, i.e. compounds containing the linkage C-N=N-C. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related to azo dyes are azo pigments, which are insoluble in water and other solvents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R−N+
2X−
where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halogen.
An azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.
Solvent Black 3 is an azo dye. It is nonfluorescent, relatively thermostable lysochrome diazo dye used for staining of neutral triglycerides and lipids on frozen sections and some lipoproteins on paraffin sections. It has the appearance of a dark brown to black powder with maximum absorption at 596–605 nm and melting point 120–124 °C. It stains blue-black.

Aniline Yellow is a yellow azo dye and an aromatic amine. It is a derivative of azobenzene. It has the appearance of an orange powder. Aniline Yellow was the first azo dye. it was first produced in 1861 by C. Mene. The second azo dye was Bismarck Brown in 1863. Aniline Yellow was commercialized in 1864 as the first commercial azo dye, a year after Aniline Black. It is manufactured from aniline.
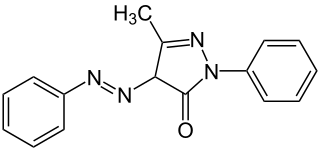
Sudan Yellow a3G, also known as Solvent Yellow 16, C.I. disperse yellow and C.I. 12700, is a yellow azo dye. It is soluble in fats and oils.
A solvent dye is a dye soluble in organic solvents. It is usually used as a solution in an organic solvent.
Aniline leather is a type of leather dyed exclusively with soluble dyes. The dye colours the leather without producing the uniform surface of a topcoat paint or insoluble pigmented sealant. The resulting product retains the hide's natural surface. Any visible variations on the surface of the undyed leather such as visible pores, scars, or other blemishes will remain visible. For this reason, only high quality leather is suitable for aniline finishing.
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. It is an organic chemical compound, consisting of a benzene ring in which an amino group is para to a nitro group. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals, gasoline, gum inhibitors, poultry medicines, and as a corrosion inhibitor.
Sudan yellow may refer to:
Aniline is an organic compound with the formula C
6H
5NH
2.
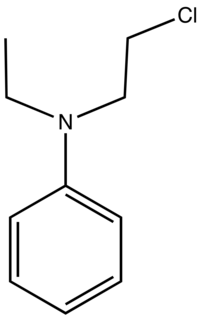
N-Ethyl-N-(2-chloroethyl)aniline is the organic compound with the formula C6H5N(Et)(CH2CH2Cl) (Et = ethyl). It is a low-melting colorless solid that is an alkylating agent. The compound is a precursor to several cationic azo dyes via reaction of the chloroethyl group with tertiary amines or pyridine followed by azo coupling. Examples of derived dyes include C. I. Basic Red 18, Maxilon Red 2GL, and Yoracryl Red 2G.
Basic Red 18 is a cationic azo dye used for coloring textiles. The chromophore is the cation, which contains many functional groups, but most prominently the quaternary ammonium center.











