Chromatography is a laboratory technique for the separation of a mixture. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. The various constituents of the mixture travel at different speeds, causing them to separate. The separation is based on differential partitioning between the mobile and stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are composed of dextran polymers (Sephadex), agarose (Sepharose), or polyacrylamide. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.

High-performance liquid chromatography is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column.
Pervaporation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.
Gel permeation chromatography (GPC) is a type of size exclusion chromatography (SEC), that separates analytes on the basis of size. The technique is often used for the analysis of polymers. As a technique, SEC was first developed in 1955 by Lathe and Ruthven. The term gel permeation chromatography can be traced back to J.C. Moore of the Dow Chemical Company who investigated the technique in 1964 and the proprietary column technology was licensed to Waters Corporation, who subsequently commercialized this technology in 1964. GPC systems and consumables are now also available from a number of manufacturers. It is often necessary to separate polymers, both to analyze them as well as to purify the desired product.

Activated carbon, also called activated charcoal, is a form of carbon processed to have small, low-volume pores that increase the surface area available for adsorption or chemical reactions. Activated is sometimes substituted with active.
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century. A wide variety of synthetic membranes is known. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane separation process. The most commonly used driving forces of a membrane process in industry are pressure and concentration gradients. The respective membrane process is therefore known as filtration. Synthetic membranes utilized in a separation process can be of different geometry and of respective flow configuration. They can also be categorized based on their application and separation regime. The best known synthetic membrane separation processes include water purification, reverse osmosis, dehydrogenation of natural gas, removal of cell particles by microfiltration and ultrafiltration, removal of microorganisms from dairy products, and Dialysis.
Hydrometallurgy is a method for obtaining metals from their ores. It is a technique within the field of extractive metallurgy involving the use of aqueous chemistry for the recovery of metals from ores, concentrates, and recycled or residual materials. Metal chemical processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy and molten salt electrometallurgy. Hydrometallurgy is typically divided into three general areas:

A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (partition) the components of a mixture into two immiscible solvent phases of different densities. Typically, one of the phases will be aqueous, and the other a lipophilic organic solvent such as ether, MTBE, dichloromethane, chloroform, or ethyl acetate. All of these solvents form a clear delineation between the two liquids. The more dense liquid, typically the aqueous phase unless the organic phase is halogenated, sinks and can be drained out through a valve away from the less dense liquid, which remains in the separatory funnel.

Ion chromatography is a chromatography process that separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of protons and electrons that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
Aqueous biphasic systems (ABS) or aqueous two-phase systems (ATPS) are clean alternatives for traditional organic-water solvent extraction systems.
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not.
Reversed-phase chromatography includes any chromatographic method that uses a hydrophobic stationary phase. RPC refers to liquid chromatography.
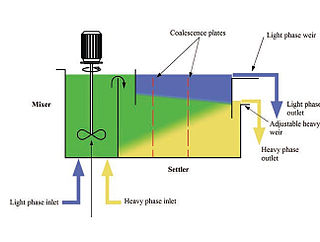
Mixer settlers are a class of mineral process equipment used in the solvent extraction process. A mixer settler consists of a first stage that mixes the phases together followed by a quiescent settling stage that allows the phases to separate by gravity.
In chemistry, phase-boundary catalysis (PBC) is a type of heterogeneous catalytic system which facilitates the chemical reaction of a particular chemical component in an immiscible phase to react on a catalytic active site located at a phase boundary. The chemical component is soluble in one phase but insoluble in the other. The catalyst for PBC has been designed in which the external part of the zeolite is hydrophobic, internally it is usually hydrophilic, notwithstanding to polar nature of some reactants. In this sense, the medium environment in this system is close to that of an enzyme. The major difference between this system and enzyme is lattice flexibility. The lattice of zeolite is rigid, whereas the enzyme is flexible.

Chloroauric acid is an inorganic compound with the chemical formula HAuCl
4. Both the trihydrate and tetrahydrate are known. It is an orange-yellow solid, a common precursor to other gold compounds and an intermediate in the purification of gold metal. Both the trihydrate and tetrahydrate are available commercially.
Leaching is the process of extracting substances from a solid by dissolving them in a liquid, either naturally or through an industrial process. In the chemical processing industry, leaching has a variety of commercial applications, including separation of metal from ore using acid, and sugar from sugar beets using hot water.
Thin layer extraction is a time-periodic reactive liquid extraction process that provides excellent mass transfer while maintaining phase separation. It is performed via a periodic batch production process that controls the time of each chemical reaction..

Extraction in chemistry is a separation process consisting in the separation of a substance from a matrix. It includes Liquid-liquid extraction, and Solid phase extraction. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte move from the water into an organic layer.

















