| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1H-Stannole | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H6Sn | |||
| Molar mass | 172.802 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
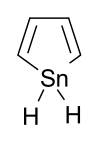
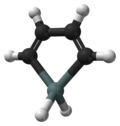
Stannole is an organotin compound with the formula (C H)4 SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of cyclopentadiene, with tin replacing the saturated carbon atom. Substituted derivatives, which have been synthesized, are also called stannoles. [1]