Mechanochemistry is the initiation of chemical reactions by mechanical phenomena. Mechanochemistry thus represents a fourth way to cause chemical reactions, complementing thermal reactions in fluids, photochemistry, and electrochemistry. Conventionally mechanochemistry focuses on the transformations of covalent bonds by mechanical force. Not covered by the topic are many phenomena: phase transitions, dynamics of biomolecules, and sonochemistry.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
A polycatenane is a chemical substance that, like polymers, is chemically constituted by a large number of units. These units are made up of concatenated rings into a chain-like structure.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of intermolecular interactions. It is an interdisciplinary academic field, bridging solid-state and supramolecular chemistry.
In organic chemistry, propellane is any member of a class of polycyclic hydrocarbons, whose carbon skeleton consists of three rings of carbon atoms sharing a common carbon–carbon covalent bond. The concept was introduced in 1966 by D. Ginsburg Propellanes with small cycles are highly strained and unstable, and are easily turned into polymers with interesting structures, such as staffanes. Partly for these reasons, they have been the object of much research.

Julius Rebek Jr. is a Hungarian-American chemist and expert on molecular self-assembly.
In polymer chemistry and materials science, the term "polymer" refers to large molecules whose structure is composed of multiple repeating units. Supramolecular polymers are a new category of polymers that can potentially be used for material applications beyond the limits of conventional polymers. By definition, supramolecular polymers are polymeric arrays of monomeric units that are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bonding, chalcogen bonding, and host–guest interaction. The direction and strength of the interactions are precisely tuned so that the array of molecules behaves as a polymer in dilute and concentrated solution, as well as in the bulk.
Jeremy Keith Morris Sanders is a British chemist and Emeritus Professor in the Department of Chemistry at the University of Cambridge. He is also Editor-in-Chief of Royal Society Open Science. He is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He served as the Pro-Vice-Chancellor for Institutional Affairs at the University of Cambridge, 2011–2015.
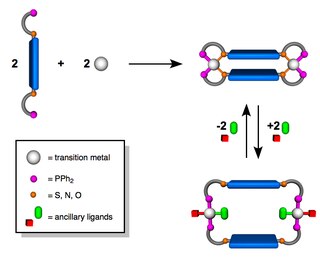
The Weak-Link Approach (WLA) is a supramolecular coordination-based assembly methodology, first introduced in 1998 by the Mirkin Group at Northwestern University. This method takes advantage of hemilabile ligands -ligands that contain both strong and weak binding moieties- that can coordinate to metal centers and quantitatively assemble into a single condensed ‘closed’ structure. Unlike other supramolecular assembly methods, the WLA allows for the synthesis of supramolecular complexes that can be modulated from rigid ‘closed’ structures to flexible ‘open’ structures through reversible binding of allosteric effectors at the structural metal centers. The approach is general and has been applied to a variety of metal centers and ligand designs including those with utility in catalysis and allosteric regulation.
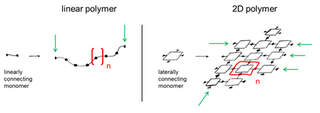
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.
Ayyappanpillai Ajayaghosh is a research scientist/academician in the domain of interdisciplinary chemistry, and the former Director of the National Institute for Interdisciplinary Science and Technology. He is known for his studies on supramolecular assemblies, organogels, photoresponsive materials, chemosensory and security materials systems and is an elected fellow of all the three major Indian science academies viz. the National Academy of Sciences, India, Indian National Science Academy and the Indian Academy of Sciences as well as The World Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Chemical Sciences in 2007. He is the first chemist to receive the Infosys Science Prize for physical sciences, awarded by the Infosys Science Foundation. He received the TWAS Prize of The World Academy of Sciences in 2013 and the Goyal prize in 2019.

Kim Kimoon is a South Korean chemist and professor in the Department of Chemistry at Pohang University of Science and Technology (POSTECH). He is the first and current director of the Center for Self-assembly and Complexity at the Institute for Basic Science. Kim has authored or coauthored 300 papers which have been cited more than 30,000 times and he holds a number of patents. His work has been published in Nature, Nature Chemistry, Angewandte Chemie, and JACS, among others. He has been a Clarivate Analytics Highly Cited Researcher in the field of chemistry in 2014, 2015, 2016.
John Isaiah Brauman is an American chemist.

Quasi-crystals are supramolecular aggregates exhibiting both crystalline (solid) properties as well as amorphous, liquid-like properties.
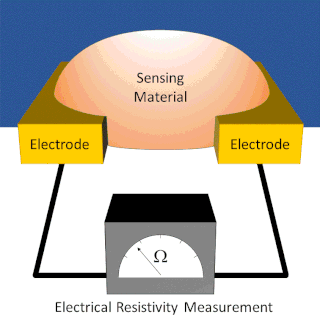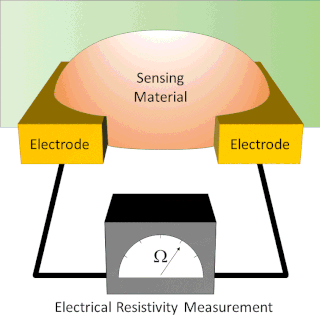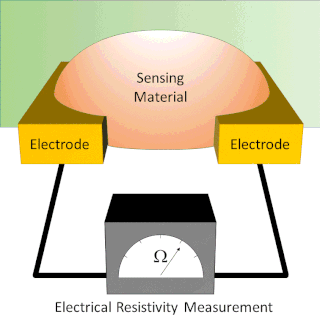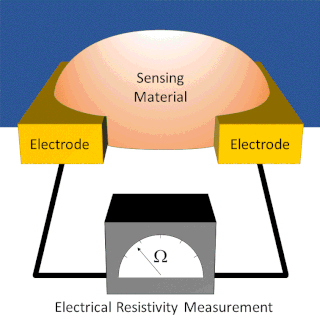
A chemiresistor is a material that changes its electrical resistance in response to changes in the nearby chemical environment. Chemiresistors are a class of chemical sensors that rely on the direct chemical interaction between the sensing material and the analyte. The sensing material and the analyte can interact by covalent bonding, hydrogen bonding, or molecular recognition. Several different materials have chemiresistor properties: semiconducting metal oxides, some conductive polymers, and nanomaterials like graphene, carbon nanotubes and nanoparticles. Typically these materials are used as partially selective sensors in devices like electronic tongues or electronic noses.

Building block is a term in chemistry which is used to describe a virtual molecular fragment or a real chemical compound the molecules of which possess reactive functional groups. Building blocks are used for bottom-up modular assembly of molecular architectures: nano-particles, metal-organic frameworks, organic molecular constructs, supra-molecular complexes. Using building blocks ensures strict control of what a final compound or a (supra)molecular construct will be.

Dmitrii "Dima" F. Perepichka is the Chair of Chemistry Department and Sir William C. MacDonald Chair Professor in Chemistry at McGill University. His research interest are primarily in the area of organic electronics. He has contributed in the understanding of structural electronics effects of organic conjugated materials at molecular, supramolecular, and macromolecular levels via the study of small molecules, supramolecular (co-)assemblies, polymers, covalent organic frameworks, and on-surface assemblies/polymers.

Guillaume De Bo is a Professor and a Royal Society University Research Fellow in the Department of Chemistry at the University of Manchester. His research is in the field of polymer mechanochemistry, where he investigates the chemistry of molecules under tension for application in synthetic chemistry, materials and mechanosensors.











