
An oligopeptide, often just called peptide, consists of two to twenty amino acids and can include dipeptides, tripeptides, tetrapeptides, and pentapeptides. Some of the major classes of naturally occurring oligopeptides include aeruginosins, cyanopeptolins, microcystins, microviridins, microginins, anabaenopeptins, and cyclamides. Microcystins are best studied, because of their potential toxicity impact in drinking water. A review of some oligopeptides found that the largest class are the cyanopeptolins (40.1%), followed by microcystins (13.4%).
Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhibitor, though it now mainly serves to boost the potency of other protease inhibitors. It may also be used in combination with other medications to treat hepatitis C and COVID-19. It is taken by mouth. Tablets of ritonavir are not bioequivalent to capsules, as the tablets may result in higher peak plasma concentrations.

Cyanotoxins are toxins produced by cyanobacteria. Cyanobacteria are found almost everywhere, but particularly in lakes and in the ocean where, under high concentration of phosphorus conditions, they reproduce exponentially to form blooms. Blooming cyanobacteria can produce cyanotoxins in such concentrations that they can poison and even kill animals and humans. Cyanotoxins can also accumulate in other animals such as fish and shellfish, and cause poisonings such as shellfish poisoning.

Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin (PST). Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic shellfish poisoning (PSP).
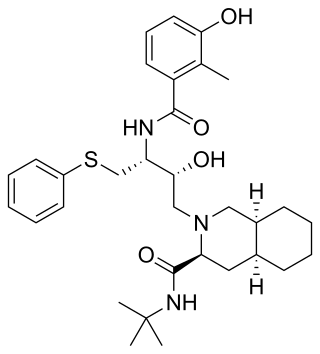
Nelfinavir, sold under the brand name Viracept, is an antiretroviral medication used in the treatment of HIV/AIDS. Nelfinavir belongs to the class of drugs known as protease inhibitors (PIs) and like other PIs is almost always used in combination with other antiretroviral drugs.

Proteasome inhibitors are drugs that block the action of proteasomes, cellular complexes that break down proteins. They are being studied in the treatment of cancer; three are approved for use in treating multiple myeloma.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and β-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.
Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV. RT is one of the most popular targets in the field of antiretroviral drug development.
Many major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate catalytic activities has an enormous therapeutic effect. Hence, inhibition of the HIV protease is one of the most important approaches for the therapeutic intervention in HIV infection and their development is regarded as major success of structure-based drug design. They are highly effective against HIV and have, since the 1990s, been a key component of anti-retroviral therapies for HIV/AIDS.
Mycosporine-like amino acids (MAAs) are small secondary metabolites produced by organisms that live in environments with high volumes of sunlight, usually marine environments. The exact number of compounds within this class of natural products is yet to be determined, since they have only relatively recently been discovered and novel molecular species are constantly being discovered; however, to date their number is around 30. They are commonly described as “microbial sunscreens” although their function is believed not to be limited to sun protection. MAAs represent high potential in cosmetics, and biotechnological applications. Indeed, their UV-absorbing properties would allow to create products derived from natural photoprotectors, potentially harmless to the environment and efficient against UV damage.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

Salinispora is a genus of obligately aerobic, gram-positive, non-acid-fast bacteria belonging to the family of Micromonosporaceae. They are heterotrophic, non-motile, and obligately grow under high osmotic/ionic-strength conditions. They are the first identified genus of gram-positive bacteria which has a high osmotic/ionic-strength requirement for survival. They are widely abundant in tropical marine sediments and were first identified in 2002. This genus of bacteria has potential biotechnological significance due to their production of novel secondary metabolites which can be used pharmaceutically.
Cyanopeptolins (CPs) are a class of oligopeptides produced by Microcystis and Planktothrix algae strains, and can be neurotoxic. The production of cyanopeptolins occurs through nonribosomal peptides synthases (NRPS).
Scytovirin is a 95-amino acid antiviral protein isolated from the cyanobacteria Scytonema varium. It has been cultured in E. coli and its structure investigated in detail. Scytovirin is thought to be produced by the bacteria to protect itself from viruses that might otherwise attack it, but as it has broad-spectrum antiviral activity against a range of enveloped viruses, scytovirin has also been found to be useful against a range of major human pathogens, most notably HIV / AIDS but also including SARS coronavirus and filoviruses such as Ebola virus and Marburg virus. While some lectins such as cyanovirin and Urtica dioica agglutinin are thought likely to be too allergenic to be used internally in humans, studies so far on scytovirin and griffithsin have not shown a similar level of immunogenicity. Scytovirin and griffithsin are currently being investigated as potential microbicides for topical use.

The microviridins are a class of serine protease inhibitors produced by various genera of cyanobacteria. Recent genome mining has shown that the biosynthetic gene cluster responsible for microviridin biosynthesis is much more prevalent, found in many species of Pseudomonadota and Bacteriodota.
Valerie J. Paul is the Director of the Smithsonian Marine Station at Fort Pierce, in Fort Pierce, FL since 2002 and the Head Scientist of the Chemical Ecology Program. She is interested in marine chemical ecology, and specializes in researching the ecology and chemistry of Cyanobacteria, blue-green algae, blooms. She has been a fellow of the American Association for the Advancement of Science since 1996, and was the chairperson of the Marine Natural Products Gordon Research Conference in 2000.

The hoiamides are a class of small molecules recently characterized from isolations of secondary metabolites of cyanobacteria that feature a triheterocyclic system. Hoiamide A and B are cyclic while hoiamide C and D are linear. Hoiamide A and B demonstrate neurotoxicity by acting on mammalian voltage gated sodium channels, while hoiamide D shows inhibition of the p53/MDM2 complex. The hoiamides are promising therapeutic targets, making their total synthesis an attractive feat.

Gallinamide A is potent and selective inhibitor of the human cysteine protease Cathepsin L1 that was first used as a moderate antimalarial agent. Gallinamide A is produced by marine cyanobacteria from Schizothrix species and Symploca sp. which have also shown to have possible anticancer agent, infectious diseases like leishmaniasis, trypanosomiasis and possible uses in Alzheimer's disease, among others.











