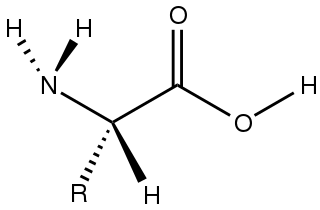
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life.

In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.

Selenium is a chemical element; it has the symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Selenocysteine is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the sulfur.

Cysteine is a semiessential proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. L‑Cysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Greek κύστη kýsti, "bladder".

Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure.
In molecular biology a selenoprotein is any protein that includes a selenocysteine amino acid residue. Among functionally characterized selenoproteins are five glutathione peroxidases (GPX) and three thioredoxin reductases, (TrxR/TXNRD) which both contain only one Sec. Selenoprotein P is the most common selenoprotein found in the plasma. It is unusual because in humans it contains 10 Sec residues, which are split into two domains, a longer N-terminal domain that contains 1 Sec, and a shorter C-terminal domain that contains 9 Sec. The longer N-terminal domain is likely an enzymatic domain, and the shorter C-terminal domain is likely a means of safely transporting the very reactive selenium atom throughout the body.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.

In biochemistry, a transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups from one molecule to another. They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
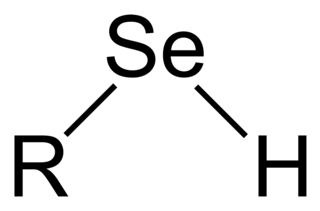
Selenols are organic compounds that contain the functional group with the connectivity C−Se−H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. A well-known selenol is the amino acid selenocysteine.

Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the GPX1 gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans.

Glutathione peroxidase 4, also known as GPX4, is an enzyme that in humans is encoded by the GPX4 gene. GPX4 is a phospholipid hydroperoxidase that protects cells against membrane lipid peroxidation.

Glutathione peroxidase 2 is an enzyme that in humans is encoded by the GPX2 gene.
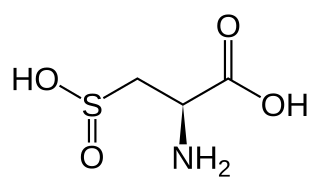
Cysteine sulfinic acid is the organic compound with the nominal formula HO2SCH2CH(NH2)CO2H. It is a rare example of an amino acid bearing a sulfinic acid functional group. It is a white solid that is soluble in water. Like most natural amino acids, it is chiral, only the L-enantiomer occurs in nature, and it exists as the zwitterion at neutral pH. It is an intermediate in cysteine metabolism. It is not a coded amino acid, but is produced post-translationally. Peptides containing the cysteine sulfinic acid residue are substrates for cysteine sulfinic acid reductase.

Glutathione peroxidase 5 (GPx-5), also known as epididymal secretory glutathione peroxidase is an enzyme that in humans is encoded by the GPX5 gene.

In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids, which are naturally encoded in the genome of organisms for the assembly of proteins. However, over 140 non-proteinogenic amino acids occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Chemically synthesized amino acids can be called unnatural amino acids. Unnatural amino acids can be synthetically prepared from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Many non-proteinogenic amino acids are important:
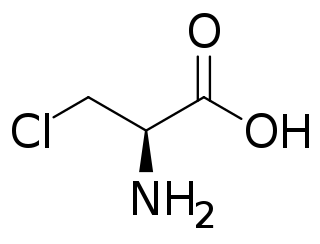
Chloroalanine (3-chloroalanine) is an unnatural amino acid with the formula ClCH2CH(NH2)CO2H. It is a white, water-soluble solid. The compound is usually derived from chlorination of serine. The compound is used in the synthesis of other amino acids by replacement of the chloride. Protected forms of the related iodoalanine are also known.

In chemistry, a selenosulfide refers to distinct classes of inorganic and organic compounds containing sulfur and selenium. The organic derivatives contain Se-S bonds, whereas the inorganic derivatives are more variable.



















