
Kevlar (para-aramid) is a strong, heat-resistant synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, the high-strength material was first used commercially in the early 1970s as a replacement for steel in racing tires. It is typically spun into ropes or fabric sheets that can be used as such, or as an ingredient in composite material components.
A monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.

A polymer is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Aramid fibers, short for aromatic polyamide, are a class of heat-resistant and strong synthetic fibers. They are used in aerospace and military applications, for ballistic-rated body armor fabric and ballistic composites, in marine cordage, marine hull reinforcement, as an asbestos substitute, and in various lightweight consumer items ranging from phone cases to tennis rackets.

Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, and thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
A polyamide is a polymer with repeating units linked by amide bonds.
Twaron is a para-aramid, high-performance yarn. It is a heat-resistant fibre, helps in ballistic protection and cut protection. Twaron was developed in the early 1970s by the Dutch company Akzo Nobel's division Enka BV, later Akzo Industrial Fibers. The research name of the para-aramid fibre was originally Fiber X, but it was soon called Arenka. Although the Dutch para-aramid fiber was developed only a little later than DuPont's Kevlar, the introduction of Twaron as a commercial product came much later than Kevlar due to financial problems at the Akzo company in the 1970s. As of 2000, Twaron had become a global material and had been integrated into the global markets. Twaron has been around for over 30 years.

p-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna.
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.

Nomex is a flame-resistant meta-aramid material developed in the early 1960s by DuPont and first marketed in 1967.

Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annually. The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid.
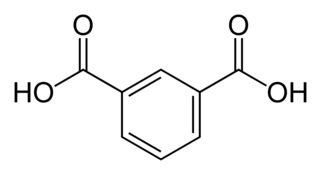
Isophthalic acid is an organic compound with the formula C6H4(CO2H)2. This colorless solid is an isomer of phthalic acid and terephthalic acid. The main industrial uses of purified isophthalic acid (PIA) are for the production of polyethylene terephthalate (PET) resin and for the production of unsaturated polyester resin (UPR) and other types of coating resins.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Polytrimethylene terephthalate (PTT), is a polyester synthesized and patented in 1941. It is produced by a method called condensation polymerization or transesterification. The two monomer units used in producing this polymer are: 1,3-propanediol and terephthalic acid or dimethyl terephthalate. Similar to polyethylene terephthalate, the PTT is used to make carpet fibers.
A repeat unit or repeating unit is a part of a polymer whose repetition would produce the complete polymer chain by linking the repeat units together successively along the chain, like the beads of a necklace.
Technora is an aramid that is useful for a variety of applications that require high strength or chemical resistance. It is a brand name of the company Teijin Aramid.

4,4′-Dichlorodiphenyl sulfone (DCDPS) is an organic compound with the formula (ClC6H4)2SO2. Classified as a sulfone, this white solid is most commonly used as a precursor to polymers that are rigid and temperature-resistant such as PES or Udel.

Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.
1,4-Bis(trichloromethyl)benzene is an organic compound with the formula C6H4(CCl3)2. A white solid, it is prepared industrially by chlorination of para-xylene. It reacts with terephthalic acid to give terephthaloyl chloride, a precursor to Kevlar. It also reacts with sulfur dioxide to give the same acid chloride and thionyl chloride. It reacts with hydrogen fluoride in 1,2-dichloroethane to form 1,4-bis(chlorodifluoromethyl)benzene in a yield of 79%.














