| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Methylpropane-2-sulfinamide | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.108.188 | ||
PubChem CID | |||
| UNII |
| ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| (CH3)3CS(O)NH2 | |||
| Molar mass | 121.20 g/mol | ||
| Appearance | white to off-white crystalline solid | ||
| Melting point | 102 to 105 °C (216 to 221 °F; 375 to 378 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
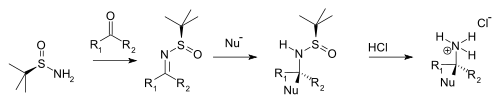
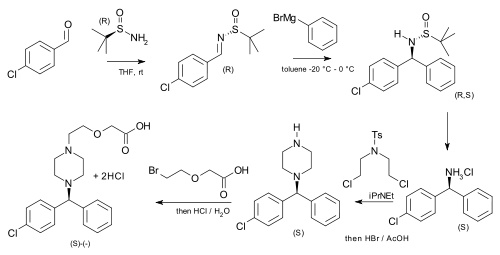
tert-Butanesulfinamide (also known as 2-methyl-2-propanesulfinamide or Ellman's sulfinamide) is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially available and are used in asymmetric synthesis as chiral auxiliaries, often as chiral ammonia equivalents for the synthesis of amines. [1] [2] [3] tert-Butanesulfinamide and the associated synthetic methodology was introduced in 1997 by Jonathan A. Ellman et al. [4]