Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.
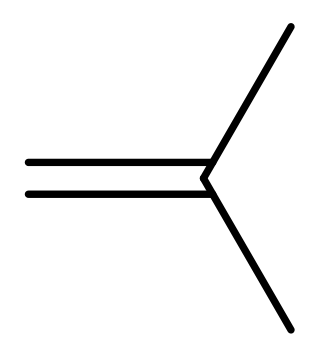
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
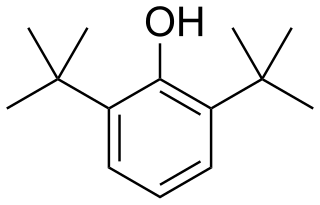
2,6-Di-tert-butylphenol is an organic compound with the structural formula 2,6-((CH3)3C)2C6H3OH. This colorless solid alkylated phenol and its derivatives are used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics. Illustrative of its usefulness, it prevents gumming in aviation fuels.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Di-tert-butyl peroxide or DTBP is an organic compound consisting of a peroxide group bonded to two tert-butyl groups. It is one of the most stable organic peroxides, due to the tert-butyl groups being bulky. It is a colorless liquid.
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. Two abbreviations for pivalic acid are t-BuC(O)OH and PivOH. The pivalyl or pivaloyl group is abbreviated t-BuC(O).
2-Methyl-2-nitrosopropane (MNP or t-nitrosobutane) is the organic compound with the formula (CH3)3CNO. It is a blue liquid that is used in chemical research as a spin trap, i.e. it binds to radicals.
tert-Butyl chloride is the organochloride with the formula (CH3)3CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds.

tert-Butylamine (also erbumine and other names) is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.
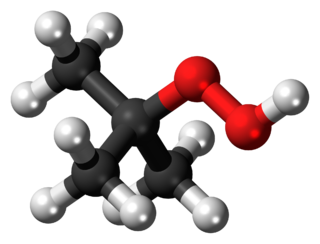
tert-Butyl hydroperoxide (tBuOOH) is the organic compound with the formula (CH3)3COOH. It is one of the most widely used hydroperoxides in a variety of oxidation processes, like the Halcon process. It is normally supplied as a 69–70% aqueous solution. Compared to hydrogen peroxide and organic peracids, tert-butyl hydroperoxide is less reactive and more soluble in organic solvents. Overall, it is renowned for the convenient handling properties of its solutions. Its solutions in organic solvents are highly stable.

tert-Butylthiol, also known as tert-butyl mercaptan (TBM), and abbreciated t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol has a strong odor. It is considered a flavoring agent.

Di-tert-butyl chromate is an alkoxide with the formula CrO2(OC(CH3)3)2. It is prepared by treatment of t-butanol with chromic anhydride. It forms red crystals at temperatures below –5 °C, above which it melts to give a red oil. The complex, which is diamagnetic, is of fundamental interest as a model for the intermediates in oxidations of alcohols by chromium(VI). This complex is stable because as a t-butyl groups lack beta-hydrogens. This complex and its analogues have tetrahedral geometry at chromium, as established by X-ray crystallography of its analogues.

tert-Butyl peroxybenzoate (TBPB) an organic compound with the formula C6H5CO3CMe3 (Me = CH3). It is the most widely produced perester; it is an ester of peroxybenzoic acid (C6H5CO3H). It is often used as a radical initiator in polymerization reactions, such as the production of LDPE from ethylene, and for crosslinking, such as for unsaturated polyester resins.

P4-t-Bu is a readily accessible chemical from the group of neutral, peralkylated sterically hindered polyaminophosphazenes, which are extremely strong bases but very weak nucleophiles, with the formula (CH3)3C−N=P(−N=P(−N(CH3)2)3)3. "t-Bu" stands for tert-butyl(CH3)3C–. "P4" stands for the fact that this molecule has 4 phosphorus atoms. P4-t-Bu can also be regarded as tetrameric triaminoiminophosphorane of the basic structure H−N=P(−NH2)3. The homologous series of P1 to P7 polyaminophosphazenes of the general formula with preferably methyl groups as R1, a methyl group or tert-butyl group as and even-numbered x between 0 and 6 (P4-t-Bu: R1 = Me, R2 = t-Bu and x = 3) has been developed by Reinhard Schwesinger; the resulting phosphazene bases are therefore also referred to as Schwesinger superbases.

Butyl methacrylate is the organic compound with the formula C4H9O2CC(CH3)=CH2. A colorless liquid, it is a common monomer for the preparation of methacrylate polymers. It is typically polymerized under free-radical conditions.
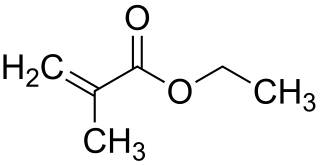
Ethyl methacrylate is the organic compound with the formula C2H5O2CC(CH3)=CH2. A colorless liquid, it is a common monomer for the preparation of acrylate polymers. It is typically polymerized under free-radical conditions.

tert-Butyl nitrite is an organic compound with the formula (CH3)3CONO. A colorless liquid, it is the tert-butyl ester of nitrous acid. It is typically employed as a solution with tert-butyl alcohol.

















