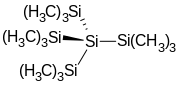Silane is an inorganic compound with chemical formula, SiH4, making it a group 14 hydride. It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon.

Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.
Chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is Silicon tetrachloride.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
Disilane is a chemical compound with chemical formula Si2H6 that was identified in 1902 by Henri Moissan and Samuel Smiles (1877–1953). Moissan and Smiles reported disilane as being among the products formed by the action of dilute acids on metal silicides. Although these reactions had been previously investigated by Friedrich Woehler and Heinrich Buff between 1857 and 1858, Moissan and Smiles were the first to explicitly identify disilane. They referred to disilane as silicoethane. Higher members of the homologous series SinH2n+2 formed in these reactions were subsequently identified by Carl Somiesky (sometimes spelled "Karl Somieski") and Alfred Stock.

Silicon tetraiodide is the chemical compound with the formula SiI4. It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
In chemistry, a hydrosilane is a tetravalent silicon compound containing an Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Especially those compounds that are liquid at room temperature are used in organosilicon chemistry. Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks. Examples include phenylsilane (PhSiH3) and triethoxysilane ((EtO)3SiH).

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(SiMe3)2. It is commonly abbreviated as LiHMDS (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.
Silylation is the introduction of a (usually) substituted silyl group (R3Si) to a molecule. The process is the basis of organosilicon chemistry.

Lithium tetrakis(pentafluorophenyl)borate is the lithium salt of the weakly coordinating anion (B(C6F5)4)−. Because of its weakly coordinating abilities, lithium tetrakis(pentafluorophenyl)borate makes it commercially valuable in the salt form in the catalyst composition for olefin polymerization reactions and in electrochemistry. It is a water-soluble compound. Its anion is closely related to the non-coordinating anion known as BARF. The tetrakis(pentafluorophenyl)borates have the advantage of operating on a one-to-one stoichiometric basis with Group IV transition metal polyolefin catalysts, unlike methylaluminoxane (MAO) which may be used in large excess.

Bis(trimethylsilyl)acetylene (BTMSA) is an organosilicon compound with the formula C2(Si(CH3)3)2. It is a colorless liquid that is soluble in organic solvents. This compound is used as a surrogate for acetylene.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.

Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3). Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).
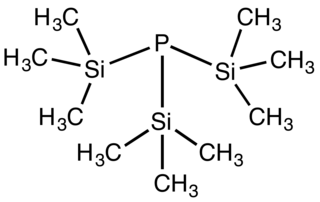
Tris(trimethylsilyl)phosphine is the organophosphorus compound with the formula P(SiMe3)3 (Me = methyl). It is a colorless liquid that ignites in air and hydrolyses readily.
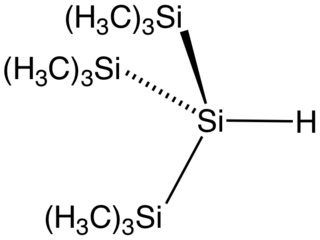
Tris(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)3SiH (where Me = CH3). It is a colorless liquid that is classified as a hydrosilane since it contains an Si-H bond. The compound is notable as having a weak Si-H bond, with a bond dissociation energy estimated at 84 kcal/mol. For comparison, the Si-H bond in trimethylsilane is 94 kcal/mol. With such a weak bond, the compound is used as a reagent to deliver hydrogen atoms. The compound has been described as an environmentally benign analogue of tributyltin hydride.
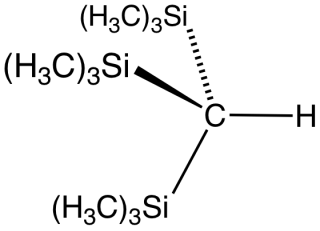
Tris(trimethylsilyl)methane is the organosilicon compound with the formula (tms)3CH (where tms = (CH3)3Si). It is a colorless liquid that is highly soluble in hydrocarbon solvents. Reaction of tris(trimethylsilyl)methane with methyl lithium gives tris(trimethylsilyl)methyllithium, called trisyllithium. Trisyllithium is useful in Petersen olefination reactions: