
In the field of molecular biology, the peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that function as transcription factors regulating the expression of genes. PPARs play essential roles in the regulation of cellular differentiation, development, and metabolism, and tumorigenesis of higher organisms.

Sudan I is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.

Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after The Second World War. Other noteworthy examples of COCs include DDT. After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides became notorious as persistent organic pollutants.
Phytol is an acyclic hydrogenated diterpene alcohol that is used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1, as well as in the fragrance industry. Its other commercial uses include cosmetics, shampoos, toilet soaps, and detergents, as well as in some cannabis distillates as a diluent or for flavoring. Its worldwide use has been estimated to be approximately 0.1–1.0 metric tons per year.
The epoxyeicosatrienoic acids or EETs are signaling molecules formed within various types of cells by the metabolism of arachidonic acid by a specific subset of Cytochrome P450 enzymes termed cytochrome P450 epoxygenases. These nonclassic eicosanoids are generally short-lived, being rapidly converted from epoxides to less active or inactive dihydroxy-eicosatrienoic acids (diHETrEs) by a widely distributed cellular enzyme, Soluble epoxide hydrolase (sEH), also termed Epoxide hydrolase 2. The EETs consequently function as transiently acting, short-range hormones; that is, they work locally to regulate the function of the cells that produce them or of nearby cells. The EETs have been most studied in animal models where they show the ability to lower blood pressure possibly by a) stimulating arterial vasorelaxation and b) inhibiting the kidney's retention of salts and water to decrease intravascular blood volume. In these models, EETs prevent arterial occlusive diseases such as heart attacks and brain strokes not only by their anti-hypertension action but possibly also by their anti-inflammatory effects on blood vessels, their inhibition of platelet activation and thereby blood clotting, and/or their promotion of pro-fibrinolytic removal of blood clots. With respect to their effects on the heart, the EETs are often termed cardio-protective. Beyond these cardiovascular actions that may prevent various cardiovascular diseases, studies have implicated the EETs in the pathological growth of certain types of cancer and in the physiological and possibly pathological perception of neuropathic pain. While studies to date imply that the EETs, EET-forming epoxygenases, and EET-inactivating sEH can be manipulated to control a wide range of human diseases, clinical studies have yet to prove this. Determination of the role of the EETS in human diseases is made particularly difficult because of the large number of EET-forming epoxygenases, large number of epoxygenase substrates other than arachidonic acid, and the large number of activities, some of which may be pathological or injurious, that the EETs possess.

The bile acid receptor (BAR), also known as farnesoid X receptor (FXR) or NR1H4, is a nuclear receptor that is encoded by the NR1H4 gene in humans.

Peroxisome proliferator- activated receptor gamma, also known as the glitazone reverse insulin resistance receptor, or NR1C3 is a type II nuclear receptor functioning as a transcription factor that in humans is encoded by the PPARG gene.
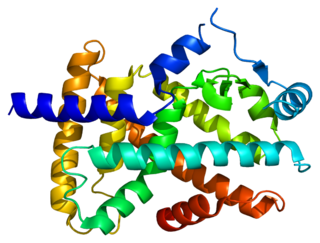
Peroxisome proliferator-activated receptor alpha (PPAR-α), also known as NR1C1, is a nuclear receptor protein functioning as a transcription factor that in humans is encoded by the PPARA gene. Together with peroxisome proliferator-activated receptor delta and peroxisome proliferator-activated receptor gamma, PPAR-alpha is part of the subfamily of peroxisome proliferator-activated receptors. It was the first member of the PPAR family to be cloned in 1990 by Stephen Green and has been identified as the nuclear receptor for a diverse class of rodent hepatocarcinogens that causes proliferation of peroxisomes.
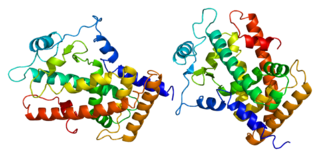
Peroxisome proliferator-activated receptor delta(PPAR-delta), or (PPAR-beta), also known as Nuclear hormone receptor 1(NUC1) is a nuclear receptor that in humans is encoded by the PPARD gene.

Free fatty acid receptor 1 (FFAR1), also known as G-protein coupled receptor 40 (GPR40), is a rhodopsin-like G-protein coupled receptor that is coded by the FFAR1 gene. This gene is located on the short arm of chromosome 19 at position 13.12. G protein-coupled receptors reside on their parent cells' surface membranes, bind any one of the specific set of ligands that they recognize, and thereby are activated to trigger certain responses in their parent cells. FFAR1 is a member of a small family of structurally and functionally related GPRs termed free fatty acid receptors (FFARs). This family includes at least three other FFARs viz., FFAR2, FFAR3, and FFAR4. FFARs bind and thereby are activated by certain fatty acids.

GW501516 is a PPARδ receptor agonist that was invented in a collaboration between Ligand Pharmaceuticals and GlaxoSmithKline in the 1990s. It entered into clinical development as a drug candidate for metabolic and cardiovascular diseases, but was abandoned in 2007 because animal testing showed that the drug caused cancer to develop rapidly in several organs.

PPAR agonists are drugs which act upon the peroxisome proliferator-activated receptor. They are used for the treatment of symptoms of the metabolic syndrome, mainly for lowering triglycerides and blood sugar.
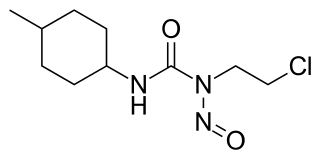
Semustine is an alkylating nitrosourea compound used in chemotherapy treatment of various types of tumours. Due to its lipophilic property, semustine can cross the blood-brain barrier for the chemotherapy of brain tumours, where it interferes with DNA replication in the rapidly-dividing tumour cells. Semustine, just as lomustine, is administered orally. Evidence has been found that treatment with semustine can cause acute leukaemia as a delayed effect in very rare cases.
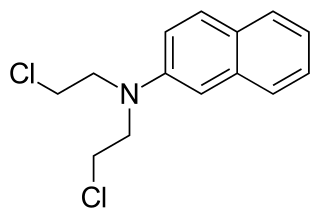
Chlornaphazine, a derivative of 2-naphthylamine, is a nitrogen mustard that was developed in the 1950s for the treatment of polycythemia and Hodgkin's disease. However, a high incidence of bladder cancers in patients receiving treatment with chlornaphthazine led to use of the drug being discontinued.

Riddelliine is a chemical compound classified as a pyrrolizidine alkaloid. It was first isolated from Senecio riddellii and is also found in a variety of plants including Jacobaea vulgaris, Senecio vulgaris, and others plants in the genus Senecio.
Lobeglitazone is an antidiabetic drug in the thiazolidinedione class of drugs. As an agonist for both PPARα and PPARγ, it works as an insulin sensitizer by binding to the PPAR receptors in fat cells and making the cells more responsive to insulin.
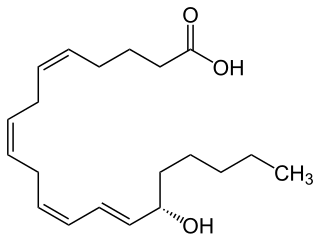
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5S,15S-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE, a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
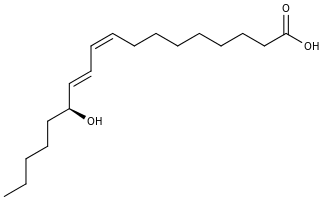
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.

20-Hydroxyeicosatetraenoic acid, also known as 20-HETE or 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid, is an eicosanoid metabolite of arachidonic acid that has a wide range of effects on the vascular system including the regulation of vascular tone, blood flow to specific organs, sodium and fluid transport in the kidney, and vascular pathway remodeling. These vascular and kidney effects of 20-HETE have been shown to be responsible for regulating blood pressure and blood flow to specific organs in rodents; genetic and preclinical studies suggest that 20-HETE may similarly regulate blood pressure and contribute to the development of stroke and heart attacks. Additionally the loss of its production appears to be one cause of the human neurological disease, Hereditary spastic paraplegia. Preclinical studies also suggest that the overproduction of 20-HETE may contribute to the progression of certain human cancers, particularly those of the breast.

Glycidamide is an organic compound with the formula H2NC(O)C2H3O. It is a colorless, oil. Structurally, it contains adjacent amides and epoxide functional groups. It is a bioactive, potentially toxic or even carcinogenic metabolite of acrylonitrile and acrylamide. It is a chiral molecule.
















