
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.

Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.

Tris(ethylenediamine)cobalt(III) chloride is an inorganic compound with the formula [Co(en)3]Cl3 (where "en" is the abbreviation for ethylenediamine). It is the chloride salt of the coordination complex [Co(en)3]3+. This trication was important in the history of coordination chemistry because of its stability and its stereochemistry. Many different salts have been described. The complex was first described by Alfred Werner who isolated this salt as yellow-gold needle-like crystals.
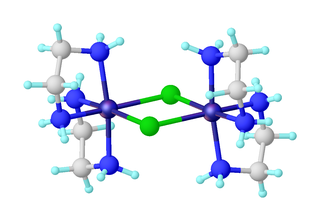
Dichlorobis(ethylenediamine)nickel(II) is the inorganic compound with the formula NiCl2(en)2, where en = ethylenediamine. The formula is deceptive: the compound is the chloride salt of the coordination complex [Ni2Cl2(en)4]2+. This blue solid is soluble in water and some polar organic solvents. It is prepared by ligand redistribution from [Ni(en)3]Cl2 · 2 H2O and hydrated nickel chloride:
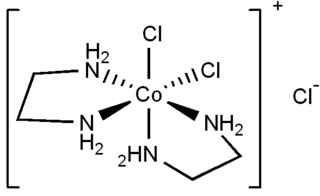
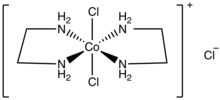
cis-Dichlorobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCl2(en)2]Cl (en = ethylenediamine). The salt consists of a cationic coordination complex and a chloride anion. It is a violet diamagnetic solid that is soluble in water. One chloride ion in this salt readily undergoes ion exchange, but the two other chlorides are less reactive, being bound to the metal center.

Chloropentamminecobalt chloride is the dichloride salt of the coordination complex [Co(NH3)5Cl]2+. It is a red-violet, diamagnetic, water-soluble salt. The compound has been of academic and historical interest.
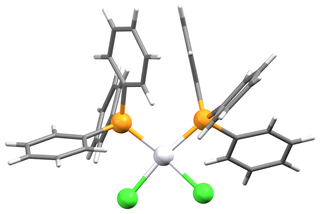
Bis(triphenylphosphine)platinum chloride is a metal phosphine complex with the formula PtCl2[P(C6H5)3]2. Cis- and trans isomers are known. The cis isomer is a white crystalline powder, while the trans isomer is yellow. Both isomers are square planar about the central platinum atom. The cis isomer is used primarily as a reagent for the synthesis of other platinum compounds.

trans-Dichlorodiammineplatinum(II) is the trans isomer of the coordination complex with the formula trans-PtCl2(NH3)2, sometimes called transplatin. It is a yellow solid with low solubility in water but good solubility in DMF. The existence of two isomers of PtCl2(NH3)2 led Alfred Werner to propose square planar molecular geometry. It belongs to the molecular symmetry point group D2h.
Nitrate chlorides are mixed anion compounds that contain both nitrate (NO3−) and chloride (Cl−) ions. Various compounds are known, including amino acid salts, and also complexes from iron group, rare-earth, and actinide metals. Complexes are not usually identified as nitrate chlorides, and would be termed chlorido nitrato complexes.

Carbonatobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCO3(en)2]Cl (en = ethylenediamine). It is a red diamagnetic solid that is soluble in water. It is the monochloride salt of a cationic carbonate complex [CoCO3(en)2]+. The chloride ion in this salt readily undergoes ion exchange. The compound is synthesized by the oxidation of a mixture of cobalt(II) chloride, lithium hydroxide, and ethylenediamine in the presence of carbon dioxide:
Cobalt compounds are chemical compounds formed by cobalt with other elements.


![UV-vis spectra of various stages in the conversion of trans-[CoCl2(en)2] to the cis isomer. Ultraviolet-visible spectroscopy of Dichlorobis(ethylenediamine)cobalt(III) chloride.png](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5c/Ultraviolet-visible_spectroscopy_of_Dichlorobis%28ethylenediamine%29cobalt%28III%29_chloride.png/220px-Ultraviolet-visible_spectroscopy_of_Dichlorobis%28ethylenediamine%29cobalt%28III%29_chloride.png)















