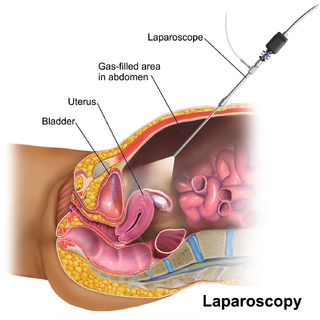
Laparoscopy is an operation performed in the abdomen or pelvis using small incisions with the aid of a camera. The laparoscope aids diagnosis or therapeutic interventions with a few small cuts in the abdomen.
Neutering, from the Latin neuter, is the removal of a non-human animal's reproductive organ, either all of it or a considerably large part. The male-specific term is castration, while spaying is usually reserved for female animals. Colloquially, both terms are often referred to as fixing. In male horses, castrating is referred to as gelding. An animal that has not been neutered is sometimes referred to as entire or intact.

Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA).

Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.
Oxetane, or 1,3-propylene oxide, is a heterocyclic organic compound with the molecular formula C
3H
6O, having a four-membered ring with three carbon atoms and one oxygen atom.
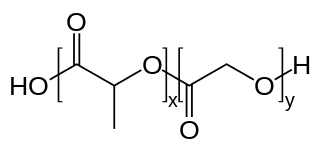
PLGA, PLG, or poly(lactic-co-glycolic) acid is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.

In organic chemistry, a carbonate ester is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R−O−C(=O)−O−R' and they are related to esters, ethers and also to the inorganic carbonates.
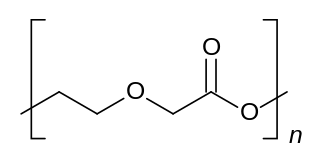
Polydioxanone or poly-p-dioxanone is a colorless, crystalline, biodegradable synthetic polymer.

A surgical anastomosis is a surgical technique used to make a new connection between two body structures that carry fluid, such as blood vessels or bowel. For example, an arterial anastomosis is used in vascular bypass and a colonic anastomosis is used to restore colonic continuity after the resection of colon cancer.

Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate is chiral, but is used as the racemic mixture in most contexts.

A trocar is a medical or veterinary device used in minimally invasive surgery. Trocars are typically made up of an awl, a cannula and often a seal. Some trocars also include a valve mechanism to allow for insufflation. Trocars are designed for placement through the chest and abdominal walls during thoracoscopic and laparoscopic surgery, and each trocar functions as a portal for the subsequent insertion of other endoscopic instruments such as grasper, scissors, stapler, electrocautery, suction tip, etc. — hence the more commonly used colloquial jargon "port". Trocars also allow passive evacuation of excess gas or fluid from organs within the body.

Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (FSO2OCH3). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence.

Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid.
A barbed suture is a type of knotless surgical suture that has barbs on its surface. While suturing tissue, these barbs penetrate inside the tissue and lock them into place, eliminating the need for knots to tie the suture. Conventional sutures rely on a surgeon's ability to tie secure knots; barbed sutures provide a knotless alternative in some surgical situations. Barbed sutures are primarily used in cosmetic surgery.
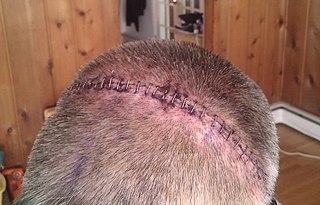
Surgical staples are specialized staples used in surgery in place of sutures to close skin wounds or connect or remove parts of the bowels or lungs. The use of staples over sutures reduces the local inflammatory response, width of the wound, and time it takes to close.
Lobectomy of the lung is a surgical operation where a lobe of the lung is removed. It is done to remove a portion of diseased lung, such as early stage lung cancer.

A surgical suture, also known as a stitch or stitches, is a medical device used to hold body tissues together and approximate wound edges after an injury or surgery. Application generally involves using a needle with an attached length of thread. There are numerous types of suture which differ by needle shape and size as well as thread material and characteristics. Selection of surgical suture should be determined by the characteristics and location of the wound or the specific body tissues being approximated.
Many opportunities exist for the application of synthetic biodegradable polymers in the biomedical area particularly in the fields of tissue engineering and controlled drug delivery. Degradation is important in biomedicine for many reasons. Degradation of the polymeric implant means surgical intervention may not be required in order to remove the implant at the end of its functional life, eliminating the need for a second surgery. In tissue engineering, biodegradable polymers can be designed such to approximate tissues, providing a polymer scaffold that can withstand mechanical stresses, provide a suitable surface for cell attachment and growth, and degrade at a rate that allows the load to be transferred to the new tissue. In the field of controlled drug delivery, biodegradable polymers offer tremendous potential either as a drug delivery system alone or in conjunction to functioning as a medical device.
Video-assisted thoracoscopic surgery (VATS) lobectomy is an approach to lung cancer surgery.

Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.
















