
An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
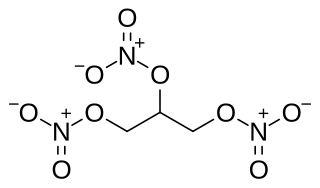
Nitroglycerin (NG), also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Invented in 1847 by Ascanio Sobrero, nitroglycerin has been used ever since as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. Since the 1880s, it has been used by the military as an active ingredient and gelatinizer for nitrocellulose in some solid propellants such as cordite and ballistite. It is a major component in double-based smokeless propellants used by reloaders. Combined with nitrocellulose, hundreds of powder combinations are used by rifle, pistol, and shotgun reloaders.

Pentaerythritol tetranitrate (PETN), also known as PENT, PENTA, TEN, corpent, or penthrite, is an explosive material. It is the nitrate ester of pentaerythritol, and is structurally very similar to nitroglycerin. Penta refers to the five carbon atoms of the neopentane skeleton. PETN is a very powerful explosive material with a relative effectiveness factor of 1.66. When mixed with a plasticizer, PETN forms a plastic explosive. Along with RDX it is the main ingredient of Semtex.

Cordite is a family of smokeless propellants developed and produced in the United Kingdom since 1889 to replace black powder as a military propellant. Like modern gunpowder, cordite is classified as a low explosive because of its slow burning rates and consequently low brisance. These produce a subsonic deflagration wave rather than the supersonic detonation wave produced by brisants, or high explosives. The hot gases produced by burning gunpowder or cordite generate sufficient pressure to propel a bullet or shell to its target, but not so quickly as to routinely destroy the barrel of the gun.
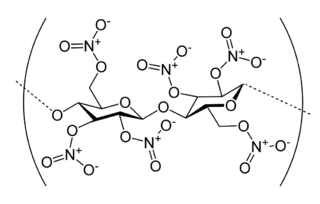
Nitrocellulose is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and sulfuric acid. One of its first major uses was as guncotton, a replacement for gunpowder as propellant in firearms. It was also used to replace gunpowder as a low-order explosive in mining and other applications. In the form of collodion it was also a critical component in an early photographic emulsion, the use of which revolutionized photography in the 1860s.
Monopropellants are propellants consisting of chemicals that release energy through exothermic chemical decomposition. The molecular bond energy of the monopropellant is released usually through use of a catalyst. This can be contrasted with bipropellants that release energy through the chemical reaction between an oxidizer and a fuel. While stable under defined storage conditions, monopropellants decompose very rapidly under certain other conditions to produce a large volume of its own energetic (hot) gases for the performance of mechanical work. Although solid deflagrants such as nitrocellulose, the most commonly used propellant in firearms, could be thought of as monopropellants, the term is usually reserved for liquids in engineering literature.
A propellant is a mass that is expelled or expanded in such a way as to create a thrust or other motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicles, the engine that expels the propellant is called a reaction engine. Although technically a propellant is the reaction mass used to create thrust, the term "propellant" is often used to describe a substance which is contains both the reaction mass and the fuel that holds the energy used to accelerate the reaction mass. For example, the term "propellant" is often used in chemical rocket design to describe a combined fuel/propellant, although the propellants should not be confused with the fuel that is used by an engine to produce the energy that expels the propellant. Even though the byproducts of substances used as fuel are also often used as a reaction mass to create the thrust, such as with a chemical rocket engine, propellant and fuel are two distinct concepts.
A plasticizer is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.

Smokeless powder is a type of propellant used in firearms and artillery that produces less smoke and less fouling when fired compared to gunpowder. The combustion products are mainly gaseous, compared to around 55% solid products for black powder. In addition, smokeless powder does not leave the thick, heavy fouling of hygroscopic material associated with black powder that causes rusting of the barrel. Despite its name, smokeless powder is not completely free of smoke; while there may be little noticeable smoke from small-arms ammunition, smoke from artillery fire can be substantial.

Otto fuel II is a monopropellant used to drive torpedoes and other weapon systems. It is not related to the Otto cycle. Otto fuel II was developed by US Navy in the 1960s for use as fuel in torpedoes. Otto fuel II was invented by Otto Reitlinger in 1963 ; it is named after Reitlinger and for being the second iteration of the fuel. The first torpedo to use it was the Mark 48 torpedo in the 1960s.

Methyl nitrate is the methyl ester of nitric acid and has the chemical formula CH3NO3. It is a colourless explosive volatile liquid.

Ethylene Glycol Dinitrate, abbreviated EGDN and NGC, also known as Nitroglycol, is a chemical compound a colorless, oily explosive liquid obtained by nitrating Ethylene Glycol. It is similar to Nitroglycerin in both manufacture and properties, though it is more volatile and less viscous. Unlike Nitroglycerine, the chemical has a perfect (0) Oxygen balance, meaning that its ideal exothermic decomposition would completely convert it to low energy CO2, H2O, and N2, with no excess unreacted O2, H2, C, or other substances, without needing to react with anything else.

Propylene glycol dinitrate (PGDN, ttup 1,2-propylene glycol dinitrate, or 1,2-propanediol dinitrate) is an organic chemical, an ester of nitric acid and propylene glycol. It is structurally similar to nitroglycerin, except that it has one fewer nitrate group. It is a characteristically and unpleasantly smelling colorless liquid, which decomposes at 121 °C, below its boiling point. It is flammable and explosive. It is shock-sensitive and burns with a clean flame producing water vapor, carbon monoxide, and nitrogen gas.

2-Nitrodiphenylamine, also called NDPA, 2-NDPA, 2NO2DPA, Sudan Yellow 1339, C.I. 10335, CI 10335, phenyl 2-nitrophenylamine, 2-nitro-N-phenylaniline, or N-phenyl-o-nitroaniline, is an organic chemical, a nitrated aromatic amine, a derivate of diphenylamine. Its chemical formula is C12H10N2O2, or C6H5NHC6H4NO2. It is a red crystalline solid, usually in form of flakes or powder, with melting point of 74-76 °C and boiling point of 346 °C. It is polar but hydrophobic.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.

1,2,4-Butanetriol trinitrate (BTTN), also called butanetriol trinitrate, is an important military propellant. It is a colorless to brown explosive liquid.
Polyvinyl nitrate (abbreviated: PVN) is a high-energy polymer with the idealized formula of [CH2CH(ONO2)]. Polyvinyl nitrate is a long carbon chain (polymer) with nitrate groups () bonded randomly along the chain. PVN is a white, fibrous solid and is soluble in polar organic solvents like acetone. PVN can be prepared by nitrating polyvinyl alcohol with an excess of nitric acid. Because PVN is also a nitrate ester like nitroglycerin (a common explosive), it exhibits energetic properties and is commonly used in explosives and propellants.

Diethylene glycol dinitrate is a nitrated alcohol ester produced by the action of concentrated nitric acid on diethylene glycol, normally with an excess of strong sulfuric acid as a dehydrating agent.
Triethylene glycol dinitrate (TEGDN) is a nitrated alcohol ester of triethylene glycol. It is used as an energetic plasticizer in explosives and propellants. Its chemical formula is O2N-O-CH2CH2-O-CH2CH2-O-CH2CH2-O-NO2. It is a pale yellow oily liquid. It is somewhat similar to nitroglycerin.
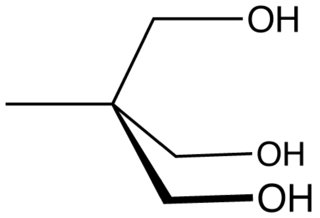
Trimethylolethane (TME) is the organic compound with the formula CH3C(CH2OH)3. This colorless solid is a triol, as it contains three hydroxy functional groups. More specifically, it features three primary alcohol groups in a compact neopentyl structure. Its esters are known for their resistance to heat, light, hydrolysis, and oxidation. More important than TME and closely related is trimethylolpropane (TMP).














