
An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
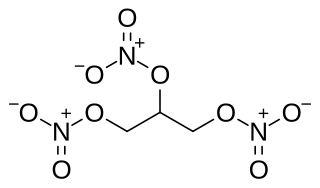
Nitroglycerin (NG), also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Discovered in 1847 by Ascanio Sobrero, nitroglycerin has been used as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. It is combined with nitrocellulose to form double-based smokeless powder, which has been used as a propellant in artillery and firearms since the 1880s.

Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Pentaerythritol tetranitrate (PETN), also known as PENT, pentyl, PENTA, TEN, corpent, or penthrite, is an explosive material. It is the nitrate ester of pentaerythritol, and is structurally very similar to nitroglycerin. Penta refers to the five carbon atoms of the neopentane skeleton. PETN is a very powerful explosive material with a relative effectiveness factor of 1.66. When mixed with a plasticizer, PETN forms a plastic explosive. Along with RDX it is the main ingredient of Semtex and C4.
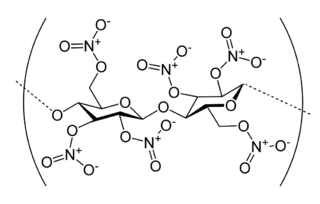
Nitrocellulose is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and sulfuric acid. One of its first major uses was as guncotton, a replacement for gunpowder as propellant in firearms. It was also used to replace gunpowder as a low-order explosive in mining and other applications. In the form of collodion it was also a critical component in an early photographic emulsion, the use of which revolutionized photography in the 1860s. In the 20th century it was adapted to automobile lacquer and adhesives.

Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste, but is toxic in high concentrations. This molecule has been observed in outer space.
Monopropellants are propellants consisting of chemicals that release energy through exothermic chemical decomposition. The molecular bond energy of the monopropellant is released usually through use of a catalyst. This can be contrasted with bipropellants that release energy through the chemical reaction between an oxidizer and a fuel. While stable under defined storage conditions, monopropellants decompose very rapidly under certain other conditions to produce a large volume of its own energetic (hot) gases for the performance of mechanical work. Although solid deflagrants such as nitrocellulose, the most commonly used propellant in firearms, could be thought of as monopropellants, the term is usually reserved for liquids in engineering literature.

A plasticizer is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.

Smokeless powder is a type of propellant used in firearms and artillery that produces less smoke and less fouling when fired compared to black powder. Because of their similar use, both the original black powder formulation and the smokeless propellant which replaced it are commonly described as gunpowder. The combustion products of smokeless powder are mainly gaseous, compared to around 55% solid products for black powder. In addition, smokeless powder does not leave the thick, heavy fouling of hygroscopic material associated with black powder that causes rusting of the barrel.

Otto fuel II is a monopropellant mixture of chiefly propylene glycol dinitrate that is used to drive torpedoes and other weapon systems. It was invented by Otto Reitlinger in 1963. Otto fuel II, sometimes known simply as Otto fuel, is not related to the Otto cycle; it is named after Reitlinger and for being the second iteration of the fuel. It was developed by the US Navy and the first torpedo to use it was the Mark 48 torpedo in the 1960s.

Mannitol hexanitrate is a powerful explosive. Physically, it is a powdery solid at normal temperature ranges, with density of 1.73 g/cm3. The chemical name is hexanitromannitol and it is also known as nitromannite, MHN, and nitromannitol, and by the trademarks Nitranitol and Mannitrin. It is more stable than nitroglycerin, and it is used in detonators.

Methyl nitrate is the methyl ester of nitric acid and has the chemical formula CH3NO3. It is a colourless explosive volatile liquid.

Ethylene glycol dinitrate, abbreviated EGDN and NGC, also known as Nitroglycol, is a colorless, oily, explosive liquid obtained by nitrating ethylene glycol. It is similar to nitroglycerine in both manufacture and properties, though it is more volatile and less viscous. Unlike nitroglycerine, the chemical has a perfect oxygen balance, meaning that its ideal exothermic decomposition would completely convert it to low energy carbon dioxide, water, and nitrogen gas, with no excess unreacted substances, without needing to react with anything else.

Propylene glycol dinitrate (PGDN, 1,2-propylene glycol dinitrate, or 1,2-propanediol dinitrate) is an organic chemical, an ester of nitric acid and propylene glycol. It is structurally similar to nitroglycerin, except that it has one fewer nitrate group. It is a characteristically and unpleasantly smelling colorless liquid, which decomposes at 121 °C, below its boiling point. It is flammable and explosive. It is shock-sensitive and burns with a clean flame producing water vapor, carbon monoxide, and nitrogen gas.

2-Nitrodiphenylamine is an organic chemical with the formula C6H5NHC6H4NO2. It is a nitrated derivative of diphenylamine. It is a red solid, usually found in form of flakes or powder. It is polar but hydrophobic.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Tetranitromethane or TNM is an organic oxidizer with chemical formula C(NO2)4. Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric acid.
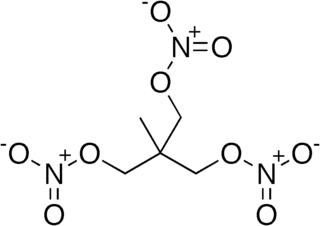
Trimethylolethane trinitrate (TMETN), also known as metriol trinitrate or nitropentaglycerin, is a nitrate ester. It is a high explosive similar to nitroglycerin. It is a transparent oily liquid, colorless to light brown. It is odorless. It is used in some solid propellants and smokeless powders as a plasticizer. Its chemical formula is CH3−C(CH2−O−NO2)3.
Triethylene glycol dinitrate (TEGDN) is an, ether, nitrated alcohol ester of triethylene glycol. It is used as an energetic plasticizer in explosives and propellants. It is a pale yellow oily liquid. It is somewhat similar to nitroglycerin.
Bulk loaded liquid propellants are an artillery technology that was pursued at the U.S. Army Research Laboratory and U.S. Naval Weapons Center from the 1950s through the 1990s. The advantages would be simpler guns and a wider range of tactical and logistic options. Better accuracy and tactical flexibility would theoretically come from standard shells with varying propellant loads, and logistic simplification by eliminating varying powder loads.















