Wilson's disease is a genetic disorder in which excess copper builds up in the body. Symptoms are typically related to the brain and liver. Liver-related symptoms include vomiting, weakness, fluid build-up in the abdomen, swelling of the legs, yellowish skin, and itchiness. Brain-related symptoms include tremors, muscle stiffness, trouble in speaking, personality changes, anxiety, and psychosis.
Xanthine oxidase is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.
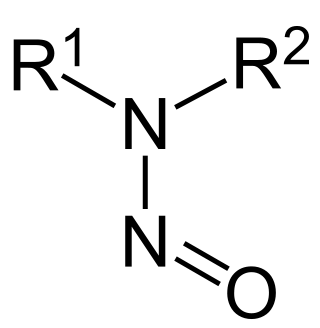
In organic chemistry, nitrosamines are organic compounds with the chemical structure R2N−N=O, where R is usually an alkyl group. They feature a nitroso group bonded to a deprotonated amine. Most nitrosamines are carcinogenic in nonhuman animals. A 2006 systematic review supports a "positive association between nitrite and nitrosamine intake and gastric cancer, between meat and processed meat intake and gastric cancer and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and gastric cancer, but is not conclusive".

Penicillamine, sold under the brand name of Cuprimine among others, is a medication primarily used for the treatment of Wilson's disease. It is also used for people with kidney stones who have high urine cystine levels, rheumatoid arthritis, and various heavy metal poisonings. It is taken by mouth.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Nitrosation is a process of converting organic compounds into nitroso derivatives, i.e., compounds containing the R-NO functionality.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.

The Bartoli indole synthesis is the chemical reaction of ortho-substituted nitroarenes and nitrosoarenes with vinyl Grignard reagents to form substituted indoles.

Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. Characteristic of its functional group, it is a dark green species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic.
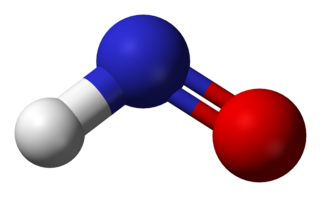
Nitroxyl or azanone is the chemical compound HNO. It is well known in the gas phase. Nitroxyl can be formed as a short-lived intermediate in the solution phase. The conjugate base, NO−, nitroxide anion, is the reduced form of nitric oxide (NO) and is isoelectronic with dioxygen. The bond dissociation energy of H−NO is 49.5 kcal/mol (207 kJ/mol), which is unusually weak for a bond to the hydrogen atom.

Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea.

Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound.
2-Methyl-2-nitrosopropane (MNP or t-nitrosobutane) is the organic compound with the formula (CH3)3CNO. It is a blue liquid that is used in chemical research as a spin trap, i.e. it binds to radicals.

Large neutral amino acids transporter small subunit 2 is a protein that in humans is encoded by the SLC7A8 gene.

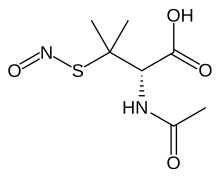
In organic chemistry, S-nitrosothiols, also known as thionitrites, are organic compounds or functional groups containing a nitroso group attached to the sulfur atom of a thiol. S-Nitrosothiols have the general formula R−S−N=O, where R denotes an organic group. Originally suggested by Ignarro to serve as intermediates in the action of organic nitrates, endogenous S-nitrosothiols were discovered by Stamler and colleagues and shown to represent a main source of NO bioactivity in vivo. More recently, S-nitrosothiols have been implicated as primary mediators of protein S-nitrosylation, the oxidative modification of cysteine thiol that provides ubiquitous regulation of protein function.

Neocuproine is a heterocyclic organic compound and chelating agent. Phenanthroline ligands were first published in the late 19th century, and the derivatives substituted at the 2 and 9 positions are among the most studied of the modified phenanthrolines.

S-Nitrosoglutathione (GSNO) is an endogenous S-nitrosothiol (SNO) that plays a critical role in nitric oxide (NO) signaling and is a source of bioavailable NO. NO coexists in cells with SNOs that serve as endogenous NO carriers and donors. SNOs spontaneously release NO at different rates and can be powerful terminators of free radical chain propagation reactions, by reacting directly with ROO• radicals, yielding nitro derivatives as end products. NO is generated intracellularly by the nitric oxide synthase (NOS) family of enzymes: nNOS, eNOS and iNOS while the in vivo source of many of the SNOs is unknown. In oxygenated buffers, however, formation of SNOs is due to oxidation of NO to dinitrogen trioxide (N2O3). Some evidence suggests that both exogenous NO and endogenously derived NO from nitric oxide synthases can react with glutathione to form GSNO.
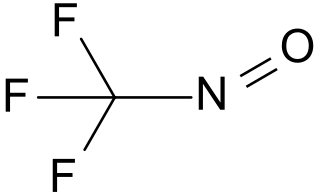
Trifluoronitrosomethane is a toxic organic compound consisting of a trifluoromethyl group covalently bound to a nitroso group. Its distinctive deep blue color is unusual for a gas.

Jonathan Solomon Stamler is an English-born American physician and scientist. He is known for his discovery of protein S-nitrosylation, the addition of a nitric oxide (NO) group to cysteine residues in proteins, as a ubiquitous cellular signal to regulate enzymatic activity and other key protein functions in bacteria, plants and animals, and particularly in transporting NO on cysteines in hemoglobin as the third gas in the respiratory cycle.

S-Nitrosotriphenylmethanethiol is the organosulfur compound with the formula (C6H5)3CSNO. It is a rare example of a nitrosothiol derivative that is robust solid at room temperature. The green compound can be produced by the reaction of triphenylmethanethiol with nitrous acid: