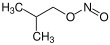
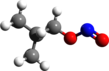
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group (the amyl in this case) is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It was first documented in 1844 and came into medical use in 1867.

Inhalants are a broad range of household and industrial chemicals whose volatile vapors or pressurized gases can be concentrated and breathed in via the nose or mouth to produce intoxication, in a manner not intended by the manufacturer. They are inhaled at room temperature through volatilization or from a pressurized container, and do not include drugs that are sniffed after burning or heating.

In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.

Club drugs, also called rave drugs or party drugs, are a loosely defined category of recreational drugs which are associated with discothèques in the 1970s and nightclubs, dance clubs, electronic dance music (EDM) parties, and raves in the 1980s to today. Unlike many other categories, such as opiates and benzodiazepines, which are established according to pharmaceutical or chemical properties, club drugs are a "category of convenience", in which drugs are included due to the locations they are consumed and/or where the user goes while under the influence of the drugs. Club drugs are generally used by adolescents and young adults.

Methylthioninium chloride, commonly called methylene blue, is a salt used as a dye and as a medication. As a medication, it is mainly used to treat methemoglobinemia by chemically reducing the ferric iron in hemoglobin to ferrous iron. Specifically, it is used to treat methemoglobin levels that are greater than 30% or in which there are symptoms despite oxygen therapy. It has previously been used for treating cyanide poisoning and urinary tract infections, but this use is no longer recommended.
Glucose-6-phosphate dehydrogenase deficiency (G6PDD), also known as favism, is the most common enzyme deficiency anemia worldwide. It is an inborn error of metabolism that predisposes to red blood cell breakdown. Most of the time, those who are affected have no symptoms. Following a specific trigger, symptoms such as yellowish skin, dark urine, shortness of breath, and feeling tired may develop. Complications can include anemia and newborn jaundice. Some people never have symptoms.

Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.

Nitrofurazone is an antimicrobial organic compound belonging to the nitrofuran class. It is most commonly used as a topical antibiotic ointment. It is effective against gram-positive bacteria, gram-negative bacteria, and can be used in the treatment of trypanosomiasis. Its use in medicine has become less frequent, as safer and more effective products have become available. Nitrofurazone is listed under California Prop 65, and has demonstrated clear evidence to be mutagenic and carcinogenic during animal studies, and has been discontinued for human use in the USA. The substance is pale yellow and crystalline. It was once widely used as an antibiotic for livestock.
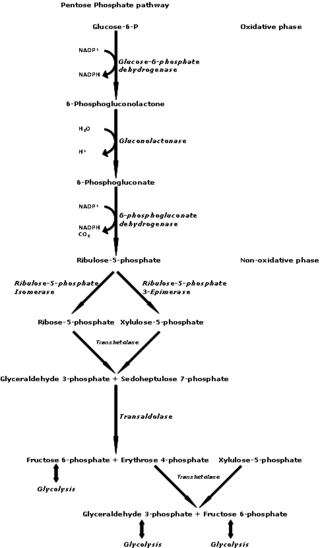
The pentose phosphate pathway is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses as well as ribose 5-phosphate, a precursor for the synthesis of nucleotides. While the pentose phosphate pathway does involve oxidation of glucose, its primary role is anabolic rather than catabolic. The pathway is especially important in red blood cells (erythrocytes). The reactions of the pathway were elucidated in the early 1950s by Bernard Horecker and co-workers.

Butyl nitrite is the organic compound with the formula CH3(CH2)3ONO. It is an alkyl nitrite made from n-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include 1-butyl nitrite, n-butyl nitrite and nitrous acid butyl ester.

The chemical compound isopropyl nitrite is an alkyl nitrite made from isopropanol. It is a clear pale yellow oil that is insoluble in water.

Cyclohexyl nitrite is an organic compound, with formula C6H11NO2. It is the ester of cyclohexanol and nitrous acid, i.e. it is an alkyl nitrite. Like amyl nitrite and butyl nitrite, it acts as an antianginal due to vasodilation. The compound is colorless, volatile liquid.
Poppers is a slang term referring to recreational drugs belonging to the alkyl nitrite family of chemical compounds. When fumes from these substances are inhaled, they act as potent vasodilators, producing mild euphoria, warmth, and dizziness. Most effects have a rapid onset and are short-acting. Its recreational use is believed to be potentially dangerous for people with heart problems, anaemia, or glaucoma. Reported adverse effects include fainting, retinal toxicity, and vision loss.

Phenylacetic acid, also known by various synonyms, is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a strong honey-like odor. Endogenously, it is a catabolite of phenylalanine. As a commercial chemical, because it can be used in the illicit production of phenylacetone, it is subject to controls in countries including the United States and China.

Glucose-6-phosphate dehydrogenase (G6PD or G6PDH) (EC 1.1.1.49) is a cytosolic enzyme that catalyzes the chemical reaction
In enzymology, a phosphogluconate dehydrogenase (decarboxylating) (EC 1.1.1.44) is an enzyme that catalyzes the chemical reaction

6-Phosphogluconate dehydrogenase deficiency, or partial deficiency, is an autosomal hereditary disease characterized by abnormally low levels of 6-phosphogluconate dehydrogenase (6PGD), a metabolic enzyme involved in the Pentose phosphate pathway. It is very important in the metabolism of red blood cells (erythrocytes). 6PDG deficiency affects less than 1% of the population, and studies suggest that there may be race variant involved in many of the reported cases. Although it is similar, 6PDG deficiency is not linked to glucose-6-phosphate dehydrogenase (G6PD) deficiency, as they are located on different chromosomes. However, a few people have had both of these metabolic diseases.

Formebolone, also known as formyldienolone, as well as 2-formyl-11α-hydroxy-17α-methyl-δ1-testosterone, is an orally active anabolic-androgenic steroid (AAS) described as an anticatabolic and anabolic drug that is or has been marketed in Spain and Italy. As an AAS, it shows some anabolic activity, though it is inferior to testosterone in terms of potency, but is said to have virtually no androgenic activity. Formebolone counteracts the catabolic effects of potent glucocorticoids like dexamethasone phosphate. A close analogue, roxibolone, shows similar antiglucocorticoid activity to formebolone but, in contrast, is devoid of activity as an AAS.
Pentyl nitrite is a chemical compound with the molecular formula, classified as an alkyl nitrite, used as an antihypertensive medicine. It is also used to treat cyanide poisoning.
Hexyl nitrite has the formula C6H13NO2 and is a nitrite and more specifically, an alkyl nitrite. It is an ester of hexanol and nitrous acid. It has the structural formula of: CH3(CH2)5ONO The CAS Registry Number is 638-51-7 and the European Community number 680-102-5. It is REACH and TSCA registered. It is also known as nitrous acid, hexyl ester. It is the aliphatic analogue of cyclohexyl nitrite.