
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It was first documented in 1844 and came into medical use in 1867.

Inhalants are a broad range of household and industrial chemicals whose volatile vapors or pressurized gases can be concentrated and breathed in via the nose or mouth to produce intoxication, in a manner not intended by the manufacturer. They are inhaled at room temperature through volatilization or from a pressurized container, and do not include drugs that are sniffed after burning or heating. For example, amyl nitrite (poppers), gasoline, nitrous oxide and toluene – a solvent widely used in contact cement, permanent markers, and certain types of glue – are considered inhalants, but smoking tobacco, cannabis, and crack cocaine are not, even though these drugs are inhaled as smoke or vapor.

Stimulants are a class of drugs that increase the activity of the brain and the spinal cord. They are used for various purposes, such as enhancing alertness, attention, motivation, cognition, mood, and physical performance. Some of the most common stimulants are caffeine, nicotine, amphetamines, cocaine, and modafinil.

Club drugs, also called rave drugs or party drugs, are a loosely defined category of recreational drugs which are associated with discothèques in the 1970s and nightclubs, dance clubs, electronic dance music (EDM) parties, and raves in the 1980s to today. Unlike many other categories, such as opiates and benzodiazepines, which are established according to pharmaceutical or chemical properties, club drugs are a "category of convenience", in which drugs are included due to the locations they are consumed and/or where the user goes while under the influence of the drugs. Club drugs are generally used by adolescents and young adults.

Isoflurane, sold under the brand name Forane among others, is a general anesthetic. It can be used to start or maintain anesthesia; however, other medications are often used to start anesthesia, due to airway irritation with isoflurane. Isoflurane is given via inhalation.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.
Verapamil, sold under various trade names, is a calcium channel blocker medication used for the treatment of high blood pressure, angina, and supraventricular tachycardia. It may also be used for the prevention of migraines and cluster headaches. It is given by mouth or by injection into a vein.

Nadolol, sold under the brand name Corgard among others, is a medication used to treat high blood pressure, heart pain, atrial fibrillation, and some inherited arrhythmic syndromes. It has also been used to prevent migraine headaches and complications of cirrhosis. It is taken orally.

Phentermine, sold under the brand name Ionamin among others, is a medication used together with diet and exercise to treat obesity. It is taken by mouth for up to a few weeks at a time, after which the benefits subside. It is also available as the combination phentermine/topiramate.
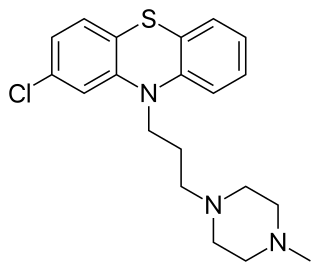
Prochlorperazine, formerly sold under the brand name Compazine among others, is a medication used to treat nausea, migraines, schizophrenia, psychosis and anxiety. It is a less preferred medication for anxiety. It may be taken by mouth, rectally, injection into a vein, or injection into a muscle.

Butyl nitrite is the organic compound with the formula CH3(CH2)3ONO. It is an alkyl nitrite made from n-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include 1-butyl nitrite, n-butyl nitrite and nitrous acid butyl ester.

The chemical compound isopropyl nitrite is an alkyl nitrite made from isopropanol. It is a clear pale yellow oil that is insoluble in water.

Cyclohexyl nitrite is an organic compound, with formula C6H11NO2. It is the ester of cyclohexanol and nitrous acid, i.e. it is an alkyl nitrite. Like amyl nitrite and butyl nitrite, it acts as an antianginal due to vasodilation. The compound is colorless, volatile liquid.
Poppers is a slang term referring to recreational drugs belonging to the alkyl nitrite family of chemical compounds. When fumes from these substances are inhaled, they act as potent vasodilators, producing mild euphoria, warmth, and dizziness. Most effects have a rapid onset and are short-acting. Reported adverse effects include retinal toxicity and vision loss. As poppers include a broad range of chemical types, their legality differs across different jurisdictions. They are often packaged under the guise of room deodorizer, leather polish, nail polish remover, or videotape head cleaner to evade anti-drug laws.

Estradiol acetate (EA), sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women. It is taken by mouth once daily or given as a vaginal ring once every three months.
Isobutyl nitrite, C4H9NO2, is an alkyl nitrite, an ester of isobutanol and nitrous acid. Its chemical structure is (CH3)2CH-CH2-ONO.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

Methylhexanamine is an indirect sympathomimetic drug invented and developed by Eli Lilly and Company and marketed as an inhaled nasal decongestant from 1948 until it was voluntarily withdrawn from the market in the 1980s.
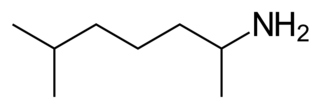
Octodrine is a stimulant drug whose pharmacology was studied in a dozen animal studies from the 1940s through the 1970s. These studies found that octodrine can increase blood pressure and cardiac output in animals. The drug was previously approved for use by the FDA as an inhalant and in Germany as an oral agent as part of a multicomponent medication, but is no longer available.
Pitolisant, sold under the brand name Wakix among others, is a medication used for the treatment of excessive daytime sleepiness in adults with narcolepsy. It is a histamine 3 (H3) receptor antagonist/inverse agonist (an antihistamine drug specific to that kind of receptors). It represents the first commercially available medication in its class, so that the US Food and Drug Administration (FDA) declares it a first-in-class medication. Pitolisant enhances the activity of histaminergic neurons in the brain that function to improve a person's wakefulness.















