
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom bonded to two organyl groups. They have the general formula R−O−R′, where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N−. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds should not be confused with azo compounds or with diazonium compounds.
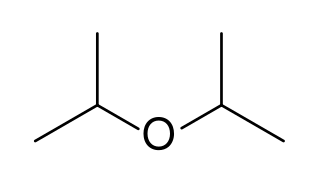
Diisopropyl ether is a secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with organic solvents. It is used as an extractant and an oxygenate gasoline additive. It is obtained industrially as a byproduct in the production of isopropanol by hydration of propylene. Diisopropyl ether is sometimes represented by the abbreviation DIPE.

Butyl nitrite is the organic compound with the formula CH3(CH2)3ONO. It is an alkyl nitrite made from n-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include 1-butyl nitrite, n-butyl nitrite and nitrous acid butyl ester.
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
The Blaise ketone synthesis is the chemical reaction of acid chlorides with organozinc compounds to give ketones.

Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.

Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
The total synthesis of quinine, a naturally-occurring antimalarial drug, was developed over a 150-year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The subject has also been attended with some controversy: Gilbert Stork published the first stereoselective total synthesis of quinine in 2001, meanwhile shedding doubt on the earlier claim by Robert Burns Woodward and William Doering in 1944, claiming that the final steps required to convert their last synthetic intermediate, quinotoxine, into quinine would not have worked had Woodward and Doering attempted to perform the experiment. A 2001 editorial published in Chemical & Engineering News sided with Stork, but the controversy was eventually laid to rest once and for all when Robert Williams and coworkers successfully repeated Woodward's proposed conversion of quinotoxine to quinine in 2007.

Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols. The reagent is often depicted as a monomer but like most hydroboranes, it is dimeric with B-H-B bridges.

tert-Butyl nitrite is an organic compound with the formula (CH3)3CONO. A colorless liquid, it is the tert-butyl ester of nitrous acid. It is typically employed as a solution with tert-butyl alcohol.
















