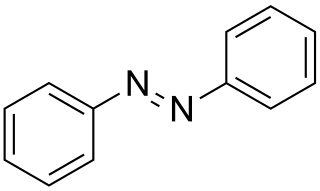
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.
Aluminium phosphate is a chemical compound. In nature it occurs as the mineral berlinite. Many synthetic forms of aluminium phosphate are known. They have framework structures similar to zeolites and some are used as catalysts, ion-exchangers or molecular sieves. Commercial aluminium phosphate gel is available.

Gallium(III) oxide is an inorganic compound and ultra-wide-bandgap semiconductor with the formula Ga2O3. It is actively studied for applications in power electronics, phosphors, and gas sensing. The compound has several polymorphs, of which the monoclinic β-phase is the most stable. The β-phase’s bandgap of 4.7–4.9 eV and large-area, native substrates make it a promising competitor to GaN and SiC-based power electronics applications and solar-blind UV photodetectors. The orthorhombic ĸ-Ga2O3 is the second most stable polymorph. The ĸ-phase has shown instability of subsurface doping density under thermal exposure. Ga2O3 exhibits reduced thermal conductivity and electron mobility by an order of magnitude compared to GaN and SiC, but is predicted to be significantly more cost-effective due to being the only wide-bandgap material capable of being grown from melt. β-Ga2O3 is thought to be radiation-hard, which makes it promising for military and space applications.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in ethereal solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.

Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.
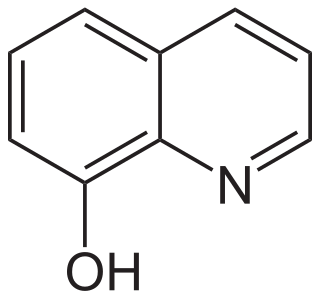
8-Hydroxyquinoline is an organic compound derived from the heterocycle quinoline. A colorless solid, its conjugate base is a chelating agent, which is used for the quantitative determination of metal ions.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
In chemistry, aluminium(I) refers to monovalent aluminium (+1 oxidation state) in both ionic and covalent bonds. Along with aluminium(II), it is an extremely unstable form of aluminium.

(Pentamethylcyclopentadienyl)aluminium(I) is an organometallic compound with the formula Al(C5Me5) ("Me" is a methyl group; CH3). The compound is often abbreviated to AlCp* or Cp*Al, where Cp* is the pentamethylcyclopentadienide anion (C5Me5−). Discovered in 1991 by Dohmeier et al., AlCp* serves as the first ever documented example of a room temperature stable monovalent aluminium compound. In its isolated form, Cp*Al exists as the tetramer [Cp*Al]4, and is a yellow crystal that decomposes at temperatures above 100 °C but also sublimes at temperatures above 140 °C.
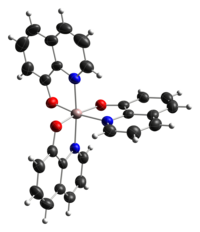
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.

Zirconium(III) bromide is an inorganic compound with the formula ZrBr3.

Zirconium(III) iodide is an inorganic compound with the formula ZrI3.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.

Tris(glycinato)cobalt(III) describes coordination complexes with the formula Co(H2NCH2CO2)3. Several isomers exist of these octahedral complexes formed between low-spin d6 Co(III) and the conjugate base of the amino acid glycine.