
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.
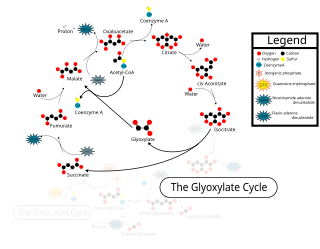
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

The enzyme argininosuccinate lyase (EC 4.3.2.1, ASL, argininosuccinase; systematic name 2-(N ω-L-arginino)succinate arginine-lyase (fumarate-forming)) catalyzes the reversible breakdown of argininosuccinate:

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
The enzyme peptidylamidoglycolate lyase catalyzes the chemical reaction
The enzyme L-erythro-3-hydroxyaspartate aldolase catalyzes the chemical reaction
The enzyme 4-hydroxy-2-oxoglutarate aldolase catalyzes the chemical reaction

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.
The enzyme malyl-CoA lyase catalyzes the chemical reaction
The enzyme oxalomalate lyase catalyzes the chemical reaction
The enzyme tartronate-semialdehyde synthase (EC 4.1.1.47) catalyzes the chemical reaction
Allantoicase is an enzyme (EC 3.5.3.4) that in humans is encoded by the ALLC gene. Allantoicase catalyzes the chemical reaction
In enzymology, an ureidoglycolate hydrolase (EC 3.5.3.19) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxyglutarate synthase (EC 2.3.3.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-ethylmalate synthase (EC 2.3.3.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-propylmalate synthase (EC 2.3.3.12) is an enzyme that catalyzes the chemical reaction

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxy-3-oxoadipate synthase (EC 2.2.1.5) is an enzyme that catalyzes the following chemical reaction:
Glyoxylate and dicarboxylate metabolism describes a variety of reactions involving glyoxylate or dicarboxylates. Glyoxylate is the conjugate base of glyoxylic acid, and within a buffered environment of known pH such as the cell cytoplasm these terms can be used almost interchangeably, as the gain or loss of a hydrogen ion is all that distinguishes them, and this can occur in the aqueous environment at any time. Likewise dicarboxylates are the conjugate bases of dicarboxylic acids, a general class of organic compounds containing two carboxylic acid groups, such as oxalic acid or succinic acid.
3-hydroxy-D-aspartate aldolase is an enzyme with systematic name 3-hydroxy-D-aspartate glyoxylate-lyase (glycine-forming). This enzyme catalyses the following chemical reaction








