
Xanthine is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine.

Banisteriopsis caapi, also known as, caapi, soul vine, or yagé (yage), is a South American liana of the family Malpighiaceae. It is commonly used as an ingredient of ayahuasca, a decoction with a long history of its entheogenic use and its status as a "plant teacher" among the Indigenous peoples of the Amazon rainforest.

β-Carboline (9H-pyrido[3,4-b]indole) represents the basic chemical structure for more than one hundred alkaloids and synthetic compounds. The effects of these substances depend on their respective substituent. Natural β-carbolines primarily influence brain functions but can also exhibit antioxidant effects. Synthetically designed β-carboline derivatives have recently been shown to have neuroprotective, cognitive enhancing and anti-cancer properties.

Harmala alkaloids are several alkaloids that increase effects of reward system neurotransmitter dopamine by acting as monoamine oxidase inhibitors (MAOIs). These alkaloids are found in the seeds of Peganum harmala, as well as leaves of tobacco and coffee beans. The alkaloids include harmine, harmaline, harmalol, and their derivatives, which have similar chemical structures, hence the name "harmala alkaloids". These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. Harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline. Harmine and harmaline are reversible inhibitors of monoamine oxidase A (RIMAs). They can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.

Peganum harmala, commonly called wild rue, Syrian rue, African rue, esfand or espand, or harmel, is a perennial, herbaceous plant, with a woody underground rootstock, of the family Nitrariaceae, usually growing in saline soils in temperate desert and Mediterranean regions. Its common English-language name came about because of a resemblance to rue. Because eating it would sicken or kill livestock, it is considered a noxious weed in a number of countries. It has become an invasive species in some regions of the western United States. The plant is popular in Middle Eastern and north African folk medicine. The alkaloids contained in the plant, including the seeds, are monoamine oxidase inhibitors.
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and Banisteriopsis caapi. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamines, making it a Reversible inhibitor of monoamine oxidase A (RIMA). Harmine does not inhibit MAO-B. Harmine is also known as banisterin, banisterine, telopathin, telepathine, leucoharmine and yagin, yageine.

Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.
An oneirogen, from the Greek ὄνειρος óneiros meaning "dream" and gen "to create", is a substance or other stimulus which produces or enhances dreamlike states of consciousness. This is characterized by an immersive dream state similar to REM sleep, which can range from realistic to alien or abstract.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Justicia adhatoda commonly known in English as Malabar nut, adulsa, adhatoda, vasa, vasaka, is native to Asia.

Tetrahydroharman(e), also known as 1-methyl-1,2,3,4-tetrahydro-β-carboline, is a general name for one of two isomers:
- (1S)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole
- Calligonine ((1R)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole)

Glaucine(1,2,9,10-TetraMethoxyAporphine, Bromcholitin, Glauvent, Tusidil, Tussiglaucin) is an aporphine alkaloid found in several different plant species in the family Papaveraceae such as Glaucium flavum, Glaucium oxylobum and Corydalis yanhusuo, and in other plants like Croton lechleri in the family Euphorbiaceae.

Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum, Annona squamosa, and Nelumbo nucifera.

Cissus quadrangularis is a perennial plant of the grape family. It is commonly known as veldt grape, winged treebine or adamant creeper. The species is native to tropical Asia, the Arabian Peninsula and much of Africa.

Harmane (harman) is a heterocyclic amine found in a variety of foods including coffee, sauces, and cooked meat. It is also present in tobacco smoke.
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).
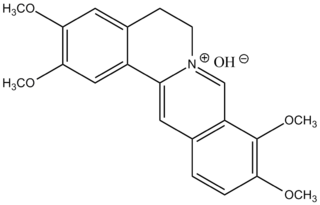
Palmatine is a protoberberine alkaloid found in several plants including Phellodendron amurense, Coptis Chinensis and Corydalis yanhusuo, Tinospora cordifolia, Tinospora sagittata, Phellodendron amurense, Stephania yunnanensis.

Vasicine (peganine) is a quinazoline alkaloid. It is found in Justicia adhatoda, after which it is named. It is additionally found in Peganum harmala.

Quinazoline alkaloids are natural products from the group of alkaloids, which are chemically derived from quinazoline. Some quinazoline alkaloids show bronchodilatory effects and stimulate respiration. An abortive effect was also found for vasicine in studies on rats and rabbits.















