
A lichen is a composite organism that arises from algae or cyanobacteria living among filaments of multiple fungi species in a mutualistic relationship. Lichens are important actors in nutrient cycling and act as producers which many higher trophic feeders feed on, such as reindeer, gastropods, nematodes, mites, and springtails. Lichens have properties different from those of their component organisms. They come in many colors, sizes, and forms and are sometimes plant-like, but are not plants. They may have tiny, leafless branches (fruticose); flat leaf-like structures (foliose); grow crust-like, adhering tightly to a surface (substrate) like a thick coat of paint (crustose); have a powder-like appearance (leprose); or other growth forms.

The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.

Ethnolichenology is the study of the relationship between lichens and people. Lichens have and are being used for many different purposes by human cultures across the world. The most common human use of lichens is for dye, but they have also been used for medicine, food and other purposes.

Queuine (Q) is a hypermodified nucleobase found in the first position of the anticodon of tRNAs specific for Asn, Asp, His, and Tyr, in most eukaryotes and prokaryotes. Because it is utilized by all eukaryotes but produced exclusively by bacteria, it is a putative vitamin.

Pulvinone, an organic compound belonging to the esters, lactones, alcohols and butenolides classes, is a yellow crystalline solid. Although the pulvinone is not a natural product, several naturally occurring hydroxylated derivatives are known. These hydroxylated pulvinones are produced by fungal species, such as the in Europe common Larch Bolete, or by moulds such as Aspergillus terreus.

Lobaria pulmonaria is a large epiphytic lichen consisting of an ascomycete fungus and a green algal partner living together in a symbiotic relationship with a cyanobacterium—a symbiosis involving members of three kingdoms of organisms. Commonly known by various names like tree lungwort, lung lichen, lung moss, lungwort lichen, oak lungs or oak lungwort, it is sensitive to air pollution and is also harmed by habitat loss and changes in forestry practices. Its population has declined across Europe and L. pulmonaria is considered endangered in many lowland areas. The species has a history of use in herbal medicines, and recent research has corroborated some medicinal properties of lichen extracts.

Vulpicida is a genus of lichenized fungi in the family Parmeliaceae. Circumscribed in 1993 to contain species formerly placed in Cetraria, the genus is widespread in Arctic to northern temperate regions, and contains six species. The genus is characterized by the presence of the secondary metabolites pulvinic acid and vulpinic acid, compounds that when combined with usnic acid, give the species their characteristic yellow and green colors.

Pseudocyphellaria is a genus of large, leafy lichens that are sometimes referred to as "specklebelly" lichens. The genus has a widespread distribution, especially in south temperate regions, and contains about 170 species. They resemble Lobaria, except that most species of Pseudocyphellaria have conspicuous pseudocyphellae on their lower surface, a characteristic that was once considered unique to this genus. Some species contain pulvinic acid-related pigments; in these species the soredia and pseudocyphellae can be bright yellow.
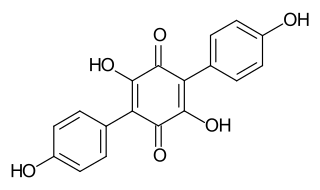
Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone.

Pulveroboletus ravenelii, commonly known as Ravenel's bolete or the powdery sulfur bolete, is a species of bolete fungus in the family Boletaceae. Described as new to science in 1853, the widely distributed species is known from Asia, Australia, North America, Central America, and South America. Mycorrhizal with oak, the fungus fruits on the ground singly, scattered, or in groups in woods. Fruit bodies (mushrooms) have convex to flat, yellowish to brownish-red caps up to 10 cm (4 in) in diameter. On the cap underside, the pore surface is bright yellow before turning dingy yellow to grayish brown with age; it stains greenish blue then grayish brown after injury. A cottony and powdery partial veil remains as a ring on the stipe. The mushrooms are edible, and have been used in traditional Chinese medicine and for mushroom dyeing.
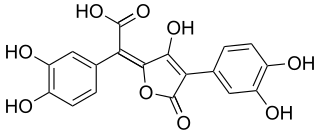
Variegatic acid is an orange pigment found in some mushrooms. It is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue quinone methide anions, specifically chinonmethid anions. It is derived from xerocomic acid, which is preceded by atromentic acid and atromentin, and its genetic basis is unknown. In its oxidized form is variegatorubin, similar to xerocomorubin.

Pulvinic acids are natural chemical pigments found in some lichens, derived biosynthetically from the aromatic amino acids phenylalanine and tyrosine, via dimerization and oxidative ring-cleavage of arylpyruvic acids, a process that also produces the related pulvinones.
Lichens of the Sierra Nevada have been little studied. A lichen is a composite organism consisting of a fungus and a photosynthetic partner growing together in a symbiotic relationship.

Symbiosis in lichens is the mutually beneficial symbiotic relationship of green algae and/or blue-green algae (cyanobacteria) living among filaments of a fungus, forming lichen.
A spot test in lichenology is a spot analysis used to help identify lichens. It is performed by placing a drop of a chemical on different parts of the lichen and noting the colour change associated with application of the chemical. The tests are routinely encountered in dichotomous keys for lichen species, and they take advantage of the wide array of lichen products produced by lichens and their uniqueness among taxa. As such, spot tests reveal the presence or absence of chemicals in various parts of a lichen. They were first proposed by the botanist William Nylander in 1866.

Candelariella vitellina is a common and widespread green-yellow to orange-yellow crustose areolate lichen that grows on rock, wood, and bark, all over the world. It grows on non-calcareous rock, wood, and bark. It often has tiny lobate areoles in the shape of lion claws. The areoles may be flat or convex. Its sexual reproduction structures (apothecia) are a 0.35–1.0 mm-wide disc, darker yellow than the thallus, rimmed with thallus-like tissue lecanorine, flat but becoming convex with age. Lichen spot tests are K+ reddish, KC−, and C−. It produces calycin, pulvinic acid, pulvinic dilactone and vulpinic acid as secondary metabolites.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.

Letharia columbiana (common name brown-eye wolf lichen, synonyms Letharia californica, Borrera columbiana) is a common lichen in subalpine forests, particularly in the Pacific Northwest of the United States, and parts of Canada. It is in the family Parmeliaceae, and the genus Letharia. Its characteristics include a bright citron color, “brown-eyes”, and rounded, irregular branches. Though previously believed to lump together several lineages such as Letharia gracilis and others, there now exists more specific characteristics to identify the species. This lichen grows on the bark of conifers a couple inches tall. L. Columbiana’s cousin, Letharia vulpina (common name wolf lichen), has similar geographical distribution and morphological features, with the major difference being the “brown-eyes” of L. columbiana.

The following outline provides an overview of and topical guide to lichens.

Umbilicaria muhlenbergii, commonly known as plated rock tripe, is a species of saxicolous (rock-dwelling, umbilicate lichen in the family Umbilicariaceae.





















