Gene knockdown is an experimental technique by which the expression of one or more of an organism's genes are reduced. The reduction can occur either through genetic modification or by treatment with a reagent such as a short DNA or RNA oligonucleotide that has a sequence complementary to either gene or an mRNA transcript.

CRISPR is a family of DNA sequences found within the genomes of prokaryotic organisms such as bacteria and archaea. These sequences are derived from DNA fragments from viruses that have previously infected the prokaryote and are used to detect and destroy DNA from similar viruses during subsequent infections. Hence these sequences play a key role in the antiviral defense system of prokaryotes.
Optogenetics is a biological technique that involves the use of light to control cells in living tissue, typically neurons, that have been genetically modified to express light-sensitive ion channels. It is a neuromodulation method that uses a combination of techniques from optics and genetics to control and monitor the activities of individual neurons in living tissue—even within freely-moving animals—and to precisely measure these manipulation effects in real-time. The key reagents used in optogenetics are light-sensitive proteins. Neuronal control is achieved using optogenetic actuators like channelrhodopsin, halorhodopsin, and archaerhodopsin, while optical recording of neuronal activities can be made with the help of optogenetic sensors for calcium (GCaMP), vesicular release (synapto-pHluorin), neurotransmitter (GluSnFRs), or membrane voltage. Control of activity is restricted to genetically defined neurons and performed in a spatiotemporal-specific manner by light.
Karl Alexander Deisseroth is the D. H. Chen Professor of Bioengineering and of Psychiatry and Behavioral Sciences at Stanford University. He earned his AB in biochemical sciences from Harvard University and his MD/PhD in neuroscience from Stanford University in 1998, and completed medical internship and psychiatry residency at Stanford Medical School. He is known for creating and developing the technologies of CLARITY and optogenetics, and for applying integrated optical and genetic strategies to study normal neural circuit function as well as dysfunction in neurological and psychiatric disease. He has led his laboratory at Stanford University since 2004, serves as an attending physician at Stanford Hospital and Clinics, and has been affiliated with the Howard Hughes Medical Institute (HHMI) since 2009. Since 2014 he is a foreign Adjunct Professor at Sweden's prestigious Karolinska medical institute.
In molecular biology, trans-activating crRNA (tracrRNA) is a small trans-encoded RNA. It was first discovered in the human pathogen Streptococcus pyogenes. In bacteria and archaea; CRISPR/Cas constitute an RNA-mediated defense system which protects against viruses and plasmids. This defensive pathway has three steps. First a copy of the invading nucleic acid is integrated into the CRISPR locus. Next, CRISPR RNAs (crRNAs) are transcribed from this CRISPR locus. The crRNAs are then incorporated into effector complexes, where the crRNA guides the complex to the invading nucleic acid and the Cas proteins degrade this nucleic acid. There are several pathways of CRISPR activation, one of which requires a tracrRNA which plays a role in the maturation of crRNA. TracrRNA is partially complementary to and base pairs with a pre-crRNA forming an RNA duplex. This is cleaved by RNase III, an RNA-specific ribonuclease, to form a crRNA/tracrRNA hybrid. This hybrid acts as a guide for the endonuclease Cas9, which cleaves the invading nucleic acid.

Jennifer Anne Doudna is an American biochemist. She is a Li Ka Shing Chancellor Chair Professor in the Department of Chemistry and the Department of Molecular and Cell Biology at the University of California, Berkeley. Doudna has been an investigator with the Howard Hughes Medical Institute (HHMI) since 1997, and since 2018 holds the position of senior investigator at the Gladstone Institutes as well as that of professor at the University of California, San Francisco.

Cas9 is an RNA-guided DNA endonuclease enzyme associated with the CRISPR adaptive immunity system in Streptococcus pyogenes, among other bacteria. S. pyogenes utilizes Cas9 to memorize and later interrogate and cleave foreign DNA, such as invading bacteriophage DNA or plasmid DNA. Cas9 performs this interrogation by unwinding foreign DNA and checking for sites complementary to the 20 basepair spacer region of the guide RNA. If the DNA substrate is complementary to the guide RNA, Cas9 cleaves the invading DNA. In this sense, the CRISPR-Cas9 mechanism has a number of parallels with the RNA interference (RNAi) mechanism in eukaryotes.

CRISPR interference (CRISPRi) is a genetic perturbation technique that allows for sequence-specific repression of gene expression in prokaryotic and eukaryotic cells. It was first developed by Stanley Qi and colleagues in the laboratories of Wendell Lim, Jonathan Weissman, and Jennifer Doudna. Sequence-specific activation of gene expression refers to CRISPR activation (CRISPRa).

Epigenome editing or Epigenome engineering is a type of genetic engineering in which the epigenome is modified at specific sites using engineered molecules targeted to those sites. Whereas gene editing involves changing the actual DNA sequence itself, epigenetic editing involves modifying and presenting DNA sequences to proteins and other DNA binding factors that influence DNA function. By "editing” epigenomic features in this manner, researchers can determine the exact biological role of an epigenetic modification at the site in question.
Protospacer adjacent motif (PAM) is a 2-6 base pair DNA sequence immediately following the DNA sequence targeted by the Cas9 nuclease in the CRISPR bacterial adaptive immune system. PAM is a component of the invading virus or plasmid, but is not a component of the bacterial CRISPR locus. Cas9 will not successfully bind to or cleave the target DNA sequence if it is not followed by the PAM sequence. PAM is an essential targeting component which distinguishes bacterial self from non-self DNA, thereby preventing the CRISPR locus from being targeted and destroyed by nuclease.
CRISPR/Cas Tools are software platforms and bioinformatics tools built to facilitate the design of guide RNAs (gRNAs) for use with the CRISPR/Cas system.

Emmanuelle Marie Charpentier is a French professor and researcher in microbiology, genetics and biochemistry. Since 2015, she has been a Director at the Max Planck Institute for Infection Biology in Berlin, Germany. In 2018, she founded an independent research institute, the Max Planck Unit for the Science of Pathogens.

The Max F. Perutz Laboratories (MFPL) are a molecular biology research centre operated jointly by the University of Vienna and the Medical University of Vienna located at the Vienna Biocenter. The institute is named after the Viennese-born biochemist and Nobel laureate Max Ferdinand Perutz. On average, MFPL hosts 50 independent research groups. MFPL scientists participate in the undergraduate curricula for students of the University of Vienna and the Medical University of Vienna.
Clustered Regularly Interspaced Short Palindromic Repeats from Prevotella and Francisella 1 or CRISPR/Cpf1 is a DNA-editing technology analogous to the CRISPR/Cas9 system. Cpf1 is an RNA-guided endonuclease of a class II CRISPR/Cas system. This acquired immune mechanism is found in Prevotella and Francisella bacteria. It prevents genetic damage from viruses. Cpf1 genes are associated with the CRISPR locus, coding for an endonuclease that use a guide RNA to find and cleave viral DNA. Cpf1 is a smaller and simpler endonuclease than Cas9, overcoming some of the CRISPR/Cas9 system limitations. CRISPR/Cpf1 could have multiple applications, including treatment of genetic illnesses and degenerative conditions.
J. Keith Joung is an American pathologist and molecular biologist who serves as Professor of Pathology at Harvard Medical School and the Associate Chief of Pathology at Massachusetts General Hospital. He is a leading figure in the field of genome editing and has pioneered the development of designer nucleases and sensitive off-target detection methods.
CRISPR-Display (CRISP-Disp) is a modification of the CRISPR/Cas9 system for genome editing. The CRISPR/Cas9 system uses a short guide RNA (sgRNA) sequence to direct a Streptococcus pyogenes Cas9 nuclease, acting as a programmable DNA binding protein, to cleave DNA at a site of interest.
Off-target genome editing refers to nonspecific and unintended genetic modifications that can arise through the use of engineered nuclease technologies such as: clustered, regularly interspaced, short palindromic repeats (CRISPR)-Cas9, transcription activator-like effector nucleases (TALEN), meganucleases, and zinc finger nucleases (ZFN). These tools use different mechanisms to bind a predetermined sequence of DNA (“target”), which they cleave, creating a double-stranded chromosomal break (DSB) that summons the cells DNA repair mechanisms and leads to site-specific modifications. If these complexes do not bind at the target, often a result of homologous sequences and/or mismatch tolerance, they will cleave off-target DSB and cause non-specific genetic modifications. Specifically, off-target effects consist of unintended point mutations, deletions, insertions inversions, and translocations.

Stanley Qi is an Assistant Professor in the Department of Bioengineering, and the Department of Chemical and Systems Biology at Stanford University. Qi lead the development of the first catalytically dead Cas9 lacking endonuclease activity (dCas9), and CRISPR interference(CRISPRi). His laboratory subsequently developed CRISPR-Genome Organization (CRISPR-GO).
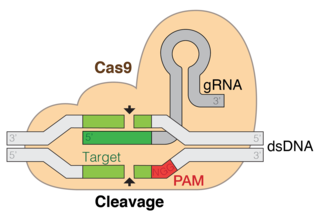
CRISPR gene editing is a method by which the genomes of living organisms may be edited. It is based on a simplified version of the bacterial CRISPR/Cas (CRISPR-Cas9) antiviral defense system. By delivering the Cas9 nuclease complexed with a synthetic guide RNA (gRNA) into a cell, the cell's genome can be cut at a desired location, allowing existing genes to be removed and/or new ones added. The Cas9-gRNA complex corresponds with the CAS III CRISPR-RNA complex in the accompanying diagram.











