The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.

Tetrahedron is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the Journal Citation Reports, Tetrahedron has a 2020 impact factor of 2.457. Tetrahedron and Elsevier, its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isopropyl-5-[(E)-2-phenylvinyl]benzene-1,3-diol, biosynthesized by the Gram-negative bacterium Photorhabdus luminescens.

The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited.
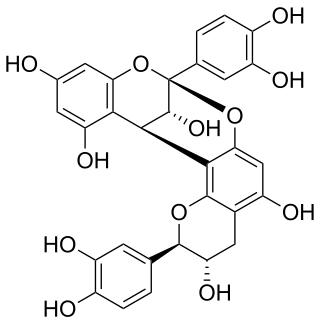
Procyanidin A1 is an A type proanthocyanidin dimer.
Hydroacylation is a type of organic reaction in which an alkene is inserted into the a formyl C-H bond. The product is a ketone. The reaction requires a metal catalyst. It is almost invariably practiced as an intramolecular reaction using homogeneous catalysts, often based on rhodium phosphines.

DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.
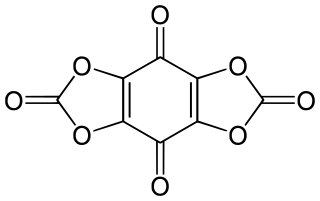
Tetrahydroxy-1,4-benzoquinone biscarbonate is a chemical compound, an oxide of carbon with formula C
8O
8. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two carbonate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and carbonic acid.
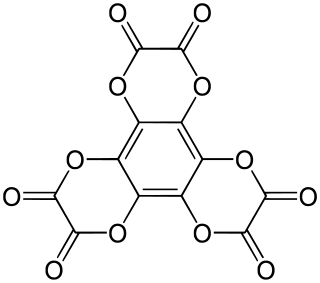
Hexahydroxybenzene trisoxalate is a chemical compound, an oxide of carbon with formula C
12O
12. Its molecule consists of a benzene core with the six hydrogen atoms replaced by three oxalate groups. It can be seen as a sixfold ester of benzenehexol and oxalic acid.

Hexahydroxybenzene triscarbonate is a chemical compound, an oxide of carbon with formula C
9O
9. Its molecular structure consists of a benzene core with the six hydrogen atoms replaced by three carbonate groups. It can be seen as a sixfold ester of hexahydroxybenzene (benzenehexol) and carbonic acid.

A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.
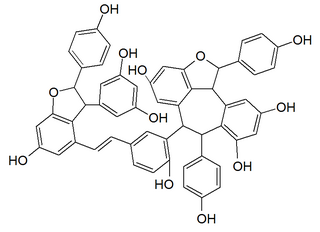
Vitisin A is a resveratrol tetramer found in plants of the genus Vitis. It is a complex of two resveratrol dimers, (+)-epsilon-viniferin and ampelopsin B.
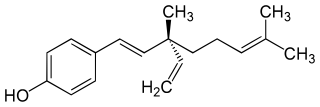
Bakuchiol is a meroterpenoid in the class terpenophenol.
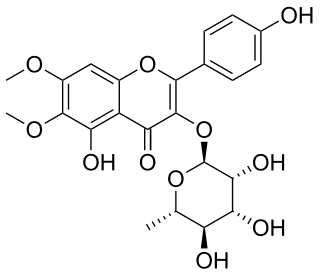
Eupalin is a flavonol. It is the eupalitin 3-O-rhamnoside. It can be isolated from Eupatorium ligustrinum.

Ampelopsin B is a stilbenoid dimer found in Ampelopsis glandulosa var. hancei.

Amurensin E is an oligostilbene found in Vitis amurensis. It is a pentamer of resveratrol.
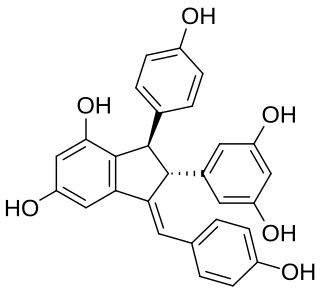
Cyphostemmin B is an oligostilbene found in Cyphostemma crotalarioides (Vitaceae). It is a resveratrol dimer.

Ampelopsis glandulosa, with common names creeper, porcelain berry, Amur peppervine, and wild grape, is an ornamental plant, native to temperate areas of Asia including China, Japan, India, Nepal, Myanmar, Vietnam, and the Philippines. It is generally similar to, and potentially confused with, grape species and other Ampelopsis species.
















