The Algar–Flynn–Oyamada reaction is a chemical reaction whereby a chalcone undergoes an oxidative cyclization to form a flavonol.
In chemistry, hydroboration refers to the addition of a hydrogen-boron bond to C-C, C-N, and C-O double bonds, as well as C-C triple bonds. This chemical reaction is useful in the organic synthesis of organic compounds. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the Nobel prize in chemistry with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates.

Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.

Tetrahedron is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the Journal Citation Reports, Tetrahedron has a 2020 impact factor of 2.457. Tetrahedron and Elsevier, its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost.
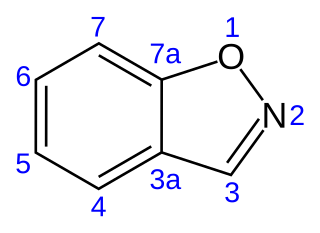
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
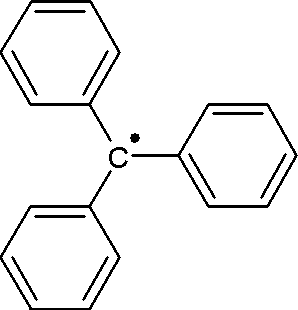
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited.
The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride. For example, toluene can be oxidized to benzaldehyde.

Ampelopsis glandulosa var. brevipedunculata, with common names creeper, porcelain berry, Amur peppervine, and wild grape, is an ornamental plant, native to temperate areas of Asia. It is generally similar to, and potentially confused with, grape species and other Ampelopsis species.
Neoflavonoids are a class of polyphenolic compounds. While flavonoids have the 2-phenylchromen-4-one backbone, neoflavonoids have the 4-phenylchromen backbone with no hydroxyl group substitution at position 2.

The Achmatowicz reaction, also known as the Achmatowicz rearrangement, is an organic synthesis in which a furan is converted to a dihydropyran. In the original publication by the Polish Chemist Osman Achmatowicz Jr. in 1971 furfuryl alcohol is reacted with bromine in methanol to 2,5-dimethoxy-2,5-dihydrofuran which rearranges to the dihydropyran with dilute sulfuric acid. Additional reaction steps, alcohol protection with methyl orthoformate and boron trifluoride) and then ketone reduction with sodium borohydride produce an intermediate from which many monosaccharides can be synthesised.

Perimycin, also known as aminomycin and fungimycin, is polyene antibiotic produced by Streptomyces coelicolor var. aminophilus. The compound exhibits antifungal properties.

Cyclotriveratrylene (CTV) is an organic compound with the formula [C6H2(OCH3)2CH2]3. It is a white solid that is soluble in organic solvents. The compound is a macrocycle and used in host–guest chemistry as a molecular host.
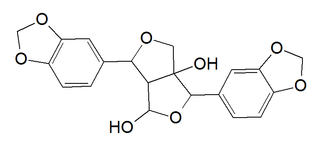
Gummadiol is a lignan hemiacetal. It can be isolated from the heartwood of Gmelina arborea.
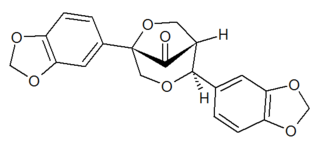
Gmelanone is a lignan found in the heartwood of Gmelina arborea. Arboreol can be transformed by acid catalysis into gmelanone.
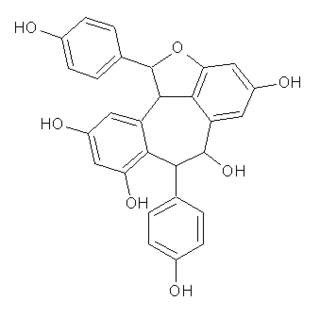
Ampelopsin A is a resveratrol dimer found in Ampelopsis glandulosa var. hancei.

Ampelopsis glandulosa is a species of plant native to China, Japan, India, Nepal, Myanmar, Vietnam, and the Philippines.

17α-Methyl-19-norprogesterone, also known as 17α-methyl-19-norpregn-4-ene-3,20-dione, is a progestin which was never marketed. It is a derivative of progesterone, and is the combined derivative of 17α-methylprogesterone and 19-norprogesterone. The drug is the parent compound of a subgroup of the 19-norprogesterone group of progestins, which includes demegestone, promegestone, and trimegestone.
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent or other similar oxaziridine reagents. This reaction mainly refers to the generation of α-hydroxy carbonyl compounds (acyloins) from ketones or esters. The reaction is carried out in a basic environment to generate the corresponding enolate from the ketone or ester. This reaction has been shown to work for amides.
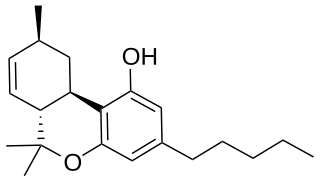
Delta-7-Tetrahydrocannabinol is a synthetic isomer of tetrahydrocannabinol. The (6aR,9S,10aR)-Δ7-THC epimer is only slightly less potent than Δ9-THC itself, while the (9R) enantiomer is much less potent.













