A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis.

The Vitaceae are a family of flowering plants, with 14 genera and around 910 known species, including common plants such as grapevines and Virginia creeper. The family name is derived from the genus Vitis.

Vaccinium vitis-idaea, the lingonberry, partridgeberry, mountain cranberry or cowberry, is a small evergreen shrub in the heath family Ericaceae, that bears edible fruit. It is native to boreal forest and Arctic tundra throughout the Northern Hemisphere, from Europe and Asia to North America. Lingonberries are picked in the wild and used to accompany a variety of dishes in Northern Baltoscandia, Russia, Canada and Alaska. Commercial cultivation is undertaken in the U.S. Pacific Northwest and in many other regions of the world.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Vitis rotundifolia, or muscadine, is a grapevine species native to the southeastern and south-central United States. The growth range extends from Florida to New Jersey coast, and west to eastern Texas and Oklahoma. It has been extensively cultivated since the 16th century. The plants are well-adapted to their native warm and humid climate; they need fewer chilling hours than better known varieties, and thrive in summer heat.

Agrobacterium is a genus of Gram-negative bacteria established by H. J. Conn that uses horizontal gene transfer to cause tumors in plants. Agrobacterium tumefaciens is the most commonly studied species in this genus. Agrobacterium is well known for its ability to transfer DNA between itself and plants, and for this reason it has become an important tool for genetic engineering.

Vitis amurensis, the Amur grape, is a species of grape native to the Asian continent. Its name comes from the Amur Valley in Russia and China.
Degeneria is a genus of flowering plants endemic to Fiji. It is the only genus in the family Degeneriaceae. The APG IV system of 2016, recognizes this family, and assigns it to the order Magnoliales in the clade magnoliids.

Vitis (grapevine) is a genus of 79 accepted species of vining plants in the flowering plant family Vitaceae. The genus is made up of species predominantly from the Northern Hemisphere. It is economically important as the source of grapes, both for direct consumption of the fruit and for fermentation to produce wine. The study and cultivation of grapevines is called viticulture.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.
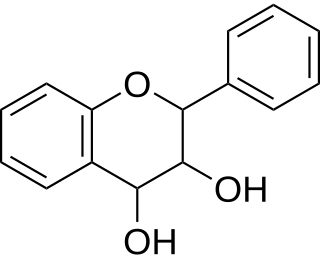
Leucoanthocyanidin (flavan-3,4-diols) are colorless chemical compounds related to anthocyanidins and anthocyanins. Leucoanthocyanins can be found in Anadenanthera peregrina and in several species of Nepenthes including N. burbidgeae, N. muluensis, N. rajah, N. tentaculata, and N. × alisaputrana.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

Vitis coignetiae, called crimson glory vine, is a plant belonging to the genus Vitis that is native to the temperate climes of Asia, where it can be found in the Russian Far East, (Sakhalin); Korea; and Japan. It was described botanically in 1883. It is called meoru (머루) in Korean and yama-budo (ヤマブドウ) in Japanese.
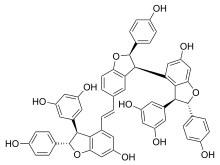
Vitisin A is a resveratrol tetramer found in plants of the genus Vitis. It is a complex of two resveratrol dimers, (+)-epsilon-viniferin and ampelopsin B.

Vitisin A is a natural phenol found in red wines. It is a pyranoanthocyanin.
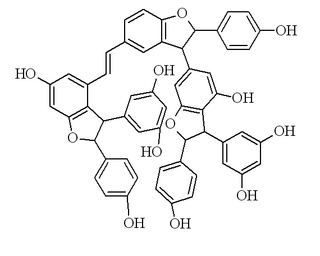
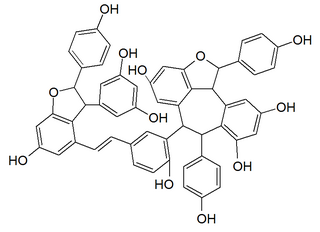
Vitisin B is a resveratrol tetramer found in plants of the genus Vitis.

Vitisin B is a natural phenol found in red wines. It is a pyranoanthocyanin.

Viniferal is a hydroxystilbenoid with an aldehyde group found in Vitis vinifera (grapevine).

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures and wine. It can also be found in Rheum maximowiczii.
Allorhizobium vitis is a plant pathogen that infects grapevines. The species is best known for causing a tumor known as crown gall disease. One of the virulent strains, A. vitis S4, is responsible both for crown gall on grapevines and for inducing a hypersensitive response in other plant species. Grapevines that have been affected by crown gall disease produce fewer grapes than unaffected plants. Though not all strains of A. vitis are tumorigenic, most strains can damage plant hosts.