
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.
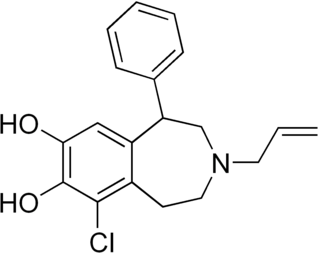
SKF-82,958 is a synthetic compound of the benzazepine class that acts as a D1/D5 receptor full agonist. SKF-82,958 and similar D1-like-selective full agonists like SKF-81,297 and 6-Br-APB produce characteristic anorectic effects, hyperactivity and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine. SKF-82,958 was also subsequently found to act as an agonist of ERα with negligible activity at ERβ, making it a subtype-selective estrogen.

Piribedil (trade names Pronoran, Trivastal Retard, Trastal, Trivastan, Clarium and others) is an antiparkinsonian agent and piperazine derivative which acts as a D2 and D3 receptor agonist. It also has α2-adrenergic antagonist properties.

β-Methylphenethylamine is an organic compound of the phenethylamine class, and a positional isomer of the drug amphetamine, with which it shares some properties. In particular, both amphetamine and β-methylphenethylamine are human TAAR1 agonists. In appearance, it is a colorless or yellowish liquid.

k-Strophanthidin is a cardenolide found in species of the genus Strophanthus. It is the aglycone of k-strophanthin, an analogue of ouabain. k-strophanthin is found in the ripe seeds of Strophanthus kombé and in the lily Convallaria.

Neuregulin 1, or NRG1, is a gene of the epidermal growth factor family that in humans is encoded by the NRG1 gene. NRG1 is one of four proteins in the neuregulin family that act on the EGFR family of receptors. Neuregulin 1 is produced in numerous isoforms by alternative splicing, which allows it to perform a wide variety of functions. It is essential for the normal development of the nervous system and the heart.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered.

(+)-BW373U86 is an opioid analgesic drug used in scientific research.
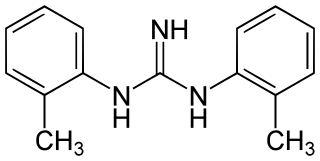
Ditolylguanidine is a sigma receptor agonist. It is somewhat selective for sigma receptors, but non-selective between the two sigma receptor subtypes, binding to both σ1 and σ2 with equal affinity. It has neuroprotective and antidepressant effects, and potentiates the effects of NMDA antagonists.
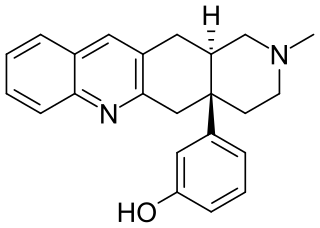
TAN-67 (SB-205,607) is an opioid drug used in scientific research that acts as a potent and selective δ-opioid agonist, selective for the δ1 subtype. It has analgesic properties and induces dopamine release in nucleus accumbens. It also protects both heart and brain tissue from hypoxic tissue damage through multiple mechanisms involving among others an interaction between δ receptors and mitochondrial K(ATP) channels.

Norbormide is a toxic compound used as a rodenticide. It has several mechanisms of action, acting as a vasoconstrictor and calcium channel blocker, but is selectively toxic to rats and has relatively low toxicity to other species, due to a species specific action of opening the permeability transition pores in rat mitochondria.

Tametraline (CP-24,441) is the parent of a series of chemical compounds investigated at Pfizer that eventually led to the development of sertraline (CP-51,974-1).

PNU-282,987 is a drug that acts as a potent and selective agonist for the α7 subtype of neural nicotinic acetylcholine receptors. In animal studies, it shows nootropic effects, and derivatives may be useful in the treatment of schizophrenia, although PNU-282,987 is not suitable for use in humans because of excessive inhibition of the hERG antitarget. PNU-282987 has been shown to initiate signaling that leads to adult neurogeneis in mammals.
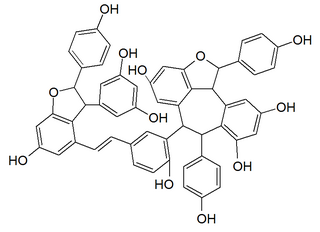
Vitisin A is a resveratrol tetramer found in plants of the genus Vitis. It is a complex of two resveratrol dimers, (+)-epsilon-viniferin and ampelopsin B.
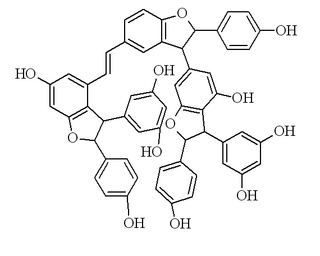
Vitisin B is a resveratrol tetramer found in plants of the genus Vitis.

Rottlerin (mallotoxin) is a polyphenol natural product isolated from the Asian tree Mallotus philippensis. Rottlerin displays a complex spectrum of pharmacology.

Isorhapontigenin is a tetrahydroxylated stilbenoid with a methoxy group. It is an isomer of rhapontigenin and an analog of resveratrol. It is found in the Chinese herb Gnetum cleistostachyum, in Gnetum parvifolium and in the seeds of the palm Aiphanes aculeata.

Oligostilbenoids are oligomeric forms of stilbenoids. Some molecules are large enough to be considered polyphenols and constitute a class of tannins.
Dihydropyridine calcium channel blockers are derivatives of 1,4-dihydropyridine that are used as L-type calcium channel blockers. They are used in the treatment of hypertension.


















