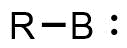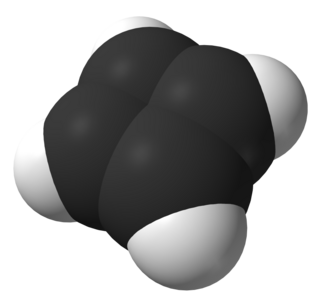
Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
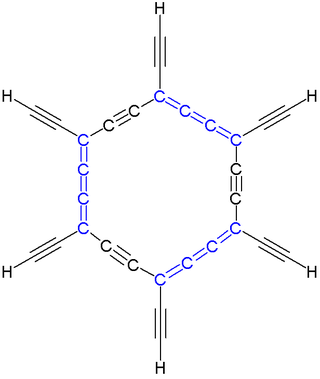
In organic chemistry, a carbo-mer (often carbo-mer or carbomer) is an expanded molecule obtained by insertion of C2 units into a given molecule. Carbo-mers differ from their templates in size but not in symmetry when each C–C single bond is replaced by an alkyne bond C-C≡C-C, each C=C double bond is replaced by an allene bond C=C=C=C, and each C≡C triple bond is replaced by C≡C-C≡C. The size of the carbo-mer continues to increase when more C2 units are inserted, so carbo-mers are also called carbon-molecules, where "n" is the number of acetylene or allene groups in an n-expansion unit. This concept, devised by Rémi Chauvin in 1995, is aimed at introducing new chemical properties for existing chemical motifs.

David A. Evans was an American chemist who was the Abbott and James Lawrence professor of chemistry at Harvard University. He was a prominent figure in the field of organic chemistry and his research focused on synthetic chemistry and total synthesis, particularly of large biologically active molecules. Among his best-known works is the development of aldol reaction methodology.

In chemistry, a molecular knot is a mechanically interlocked molecular architecture that is analogous to a macroscopic knot. Naturally-forming molecular knots are found in organic molecules like DNA, RNA, and proteins. It is not certain that naturally occurring knots are evolutionarily advantageous to nucleic acids or proteins, though knotting is thought to play a role in the structure, stability, and function of knotted biological molecules. The mechanism by which knots naturally form in molecules, and the mechanism by which a molecule is stabilized or improved by knotting, is ambiguous. The study of molecular knots involves the formation and applications of both naturally occurring and chemically synthesized molecular knots. Applying chemical topology and knot theory to molecular knots allows biologists to better understand the structures and synthesis of knotted organic molecules.
In chemistry, mechanically interlocked molecular architectures (MIMAs) are molecules that are connected as a consequence of their topology. This connection of molecules is analogous to keys on a keychain loop. The keys are not directly connected to the keychain loop but they cannot be separated without breaking the loop. On the molecular level, the interlocked molecules cannot be separated without the breaking of the covalent bonds that comprise the conjoined molecules; this is referred to as a mechanical bond. Examples of mechanically interlocked molecular architectures include catenanes, rotaxanes, molecular knots, and molecular Borromean rings. Work in this area was recognized with the 2016 Nobel Prize in Chemistry to Bernard L. Feringa, Jean-Pierre Sauvage, and J. Fraser Stoddart.

In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene rings which have been linearly fused. They follow the general molecular formula C4n+2H2n+4.
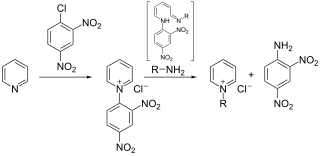
The Zincke reaction is an organic reaction, named after Theodor Zincke, in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine.
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being collectively awarded one half of the 2001 Nobel Prize in Chemistry.
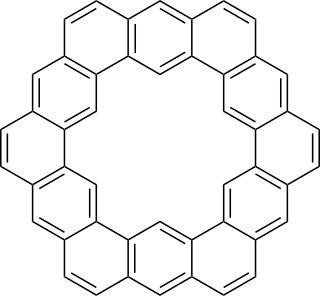
Kekulene is a polycyclic aromatic hydrocarbon which consists of 12 fused benzene rings arranged in a circle. It is therefore classified as a [12]-circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.

Harry Laurence Anderson is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems, exploration of the extraordinary physical properties of large pi-conjugated systems, and synthesis of cyclo[18]carbon. He is a Professor of Chemistry at Keble College, Oxford.

Kim Kimoon is a South Korean chemist and professor in the Department of Chemistry at Pohang University of Science and Technology (POSTECH). He is the first and current director of the Center for Self-assembly and Complexity at the Institute for Basic Science. Kim has authored or coauthored 300 papers which have been cited more than 30,000 times and he holds a number of patents. His work has been published in Nature, Nature Chemistry, Angewandte Chemie, and JACS, among others. He has been a Clarivate Analytics Highly Cited Researcher in the field of chemistry in 2014, 2015, 2016.
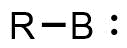
A borylene is the boron analogue of a carbene. The general structure is R-B: with R an organic moiety and B a boron atom with two unshared electrons. Borylenes are of academic interest in organoboron chemistry. A singlet ground state is predominant with boron having two vacant sp2 orbitals and one doubly occupied one. With just one additional substituent the boron is more electron deficient than the carbon atom in a carbene. For this reason stable borylenes are more uncommon than stable carbenes. Some borylenes such as boron monofluoride (BF) and boron monohydride (BH) the parent compound also known simply as borylene, have been detected in microwave spectroscopy and may exist in stars. Other borylenes exist as reactive intermediates and can only be inferred by chemical trapping.
A cycloparaphenylene is a molecule that consists of several benzene rings connected by covalent bonds in the para positions to form a hoop- or necklace-like structure. Its chemical formula is [C6H4]n or C
6nH
4n Such a molecule is usually denoted [n]CPP where n is the number of benzene rings.
The phosphaethynolate anion, also referred to as PCO, is the phosphorus-containing analogue of the cyanate anion with the chemical formula [PCO]− or [OCP]−. The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.
Xue-Min Cheng is a medicinal chemist, author and pharmaceutical executive best known as the co-author of The Logic of Chemical Synthesis, which formalized retrosynthesis. The concept for this Elias J. Corey won the 1990 Nobel Prize in Chemistry.

Nontrigonal pnictogen compounds refer to tricoordinate trivalent pnictogen compounds that are not of typical trigonal pyramidal molecular geometry. By virtue of their geometric constraint, these compounds exhibit distinct electronic structures and reactivities, which bestow on them potential to provide unique nonmetal platforms for bond cleavage reactions.
René Peters is a German chemist and since 2008 Professor of Organic Chemistry at the University of Stuttgart.