
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of molecules, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion, achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the many-body problem exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical experiments, it can occasionally predict unobserved chemical phenomena.
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of molecules, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed wave functions as well as to observable properties such as structures, spectra, and thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics.
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic behavior of nature from the behavior of such ensembles.

Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface of potential energy, molecular orbitals, orbital interactions, and molecule activation.
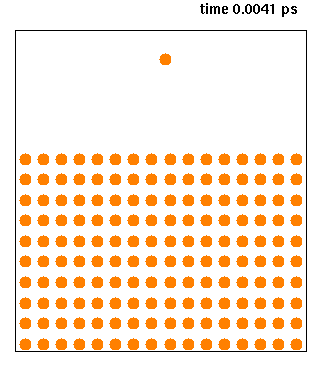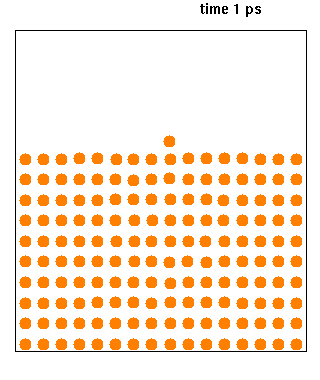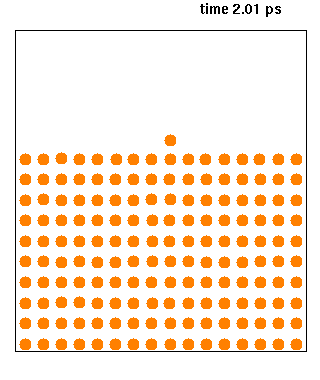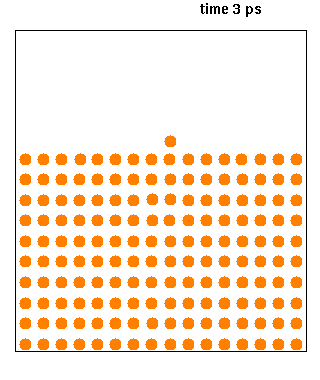
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are often calculated using interatomic potentials or molecular mechanical force fields. The method is applied mostly in chemical physics, materials science, and biophysics.

Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials science to study molecular systems ranging from small chemical systems to large biological molecules and material assemblies. The simplest calculations can be performed by hand, but inevitably computers are required to perform molecular modelling of any reasonably sized system. The common feature of molecular modelling methods is the atomistic level description of the molecular systems. This may include treating atoms as the smallest individual unit, or explicitly modelling protons and neutrons with its quarks, anti-quarks and gluons and electrons with its photons.
In chemical kinetics, a reaction rate constant or reaction rate coefficient is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants.

In organic chemistry, cheletropic reactions, also known as chelotropic reactions, are a type of pericyclic reaction. Specifically, cheletropic reactions are a subclass of cycloadditions. The key distinguishing feature of cheletropic reactions is that on one of the reagents, both new bonds are being made to the same atom.
Amsterdam Density Functional (ADF) is a program for first-principles electronic structure calculations that makes use of density functional theory (DFT). ADF was first developed in the early seventies by the group of E. J. Baerends from the Vrije Universiteit in Amsterdam, and by the group of T. Ziegler from the University of Calgary. Nowadays many other academic groups are contributing to the software. Software for Chemistry & Materials (SCM), formerly known as Scientific Computing & Modelling is a spin-off company from the Baerends group. SCM has been coordinating the development and distribution of ADF since 1995. Together with the rise in popularity of DFT in the nineties, ADF has become a popular computational chemistry software package used in the industrial and academic research. ADF excels in spectroscopy, transition metals, and heavy elements problems. A periodic structure counterpart of ADF named BAND is available to study bulk crystals, polymers, and surfaces. The Amsterdam Modeling Suite has expanded beyond DFT since 2010, with the semi-empirical MOPAC code, the Quantum_ESPRESSO plane wave code, a density-functional based tight binding (DFTB) module, a reactive force field module ReaxFF, and an implementation of Klamt's COSMO-RS method, which also includes COSMO-SAC, UNIFAC, and QSPR.

Robert Goulston Gilbert is a polymer chemist whose most significant contributions have been in the field of emulsion polymerisation. In 1970, he gained his PhD from the Australian National University, and worked at the University of Sydney from then until 2006. In 1982, he was elected a fellow of the Royal Australian Chemical Institute; in 1994, he was elected a fellow of the Australian Academy of Science. In 1992, he was appointed full professor, and in 1999 he started the Key Centre for Polymer Colloids, funded by the Australian Research Council, the University and industry. He has served in leadership roles in the International Union of Pure and Applied Chemistry (IUPAC), the world ‘governing body’ of chemistry. He was founding chair (1987–98) of the IUPAC Working Party on the Modelling of Kinetics Processes of Polymerisation, of which he remains a member, and is a member of the IUPAC scientific task groups on starch molecular weight measurements, and terminology. He was vice-president (1996–97) and president (1998–2001) of the IUPAC Macromolecular Division, and secretary of the International Polymer Colloids Group (1997–2001). As of 2007, he is Research Professor at the Centre of Nutrition and Food Science, University of Queensland, where his research program concentrates on the relations between starch structure and nutrition.
The COCO Simulator is a free-of-charge, non-commercial, graphical, modular and CAPE-OPEN compliant, steady-state, sequential simulation process modeling environment. It was originally intended as a test environment for CAPE-OPEN modeling tools but now provides free chemical process simulation for students. It is an open flowsheet modeling environment allowing anyone to add new unit operations or thermodynamics packages.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
CALPHAD stands for CALculation of PHAse Diagrams, a methodology introduced in 1970 by Larry Kaufman. An equilibrium phase diagram is usually a diagram with axes for temperature and composition of a chemical system. It shows the regions where substances or solutions are stable and regions where two or more of them coexist. Phase diagrams are a very powerful tool for predicting the state of a system under different conditions and were initially a graphical method to rationalize experimental information on states of equilibrium. In complex systems, computational methods such as CALPHAD are employed to model thermodynamic properties for each phase and simulate multicomponent phase behavior. The CALPHAD approach is based on the fact that a phase diagram is a manifestation of the equilibrium thermodynamic properties of the system, which are the sum of the properties of the individual phases. It is thus possible to calculate a phase diagram by first assessing the thermodynamic properties of all the phases in a system.

In theoretical chemistry, an energy profile is a theoretical representation of a chemical reaction or process as a single energetic pathway as the reactants are transformed into products. This pathway runs along the reaction coordinate, which is a parametric curve that follows the pathway of the reaction and indicates its progress; thus, energy profiles are also called reaction coordinate diagrams. They are derived from the corresponding potential energy surface (PES), which is used in computational chemistry to model chemical reactions by relating the energy of a molecule(s) to its structure.
Process simulation is used for the design, development, analysis, and optimization of technical process of simulation of processes such as: chemical plant s, chemical processes, environmental systems, power stations, complex manufacturing operations, biological processes, and similar technical functions.
Chemical WorkBench is a proprietary simulation software tool aimed at the reactor scale kinetic modeling of homogeneous gas-phase and heterogeneous processes and kinetic mechanism development. It can be effectively used for the modeling, optimization, and design of a wide range of industrially and environmentally important chemistry-loaded processes. Chemical WorkBench is a modeling environment based on advanced scientific approaches, complementary databases, and accurate solution methods. Chemical WorkBench is developed and distributed by Kintech Lab.
Quantemol Ltd is based in University College London initiated by Professor Jonathan Tennyson FRS and Dr. Daniel Brown in 2004. The company initially developed a unique software tool, Quantemol-N, which provides full accessibility to the highly sophisticated UK molecular R-matrix codes, used to model electron polyatomic molecule interactions. Since then Quantemol has widened to further types of simulation, with plasmas and industrial plasma tools, in Quantemol-VT in 2013 and launched in 2016 a sustainable database Quantemol-DB, representing the chemical and radiative transport properties of a wide range of plasmas.
The Geochemist's Workbench (GWB) is an integrated set of interactive software tools for solving a range of problems in aqueous chemistry. The graphical user interface simplifies the use of the geochemical code.
Geochemical modeling or theoretical geochemistry is the practice of using chemical thermodynamics, chemical kinetics, or both, to analyze the chemical reactions that affect geologic systems, commonly with the aid of a computer. It is used in high-temperature geochemistry to simulate reactions occurring deep in the Earth's interior, in magma, for instance, or to model low-temperature reactions in aqueous solutions near the Earth's surface, the subject of this article.
COSMO-RS is a quantum chemistry based equilibrium thermodynamics method with the purpose of predicting chemical potentials µ in liquids. It processes the screening charge density σ on the surface of molecules to calculate the chemical potential µ of each species in solution. Perhaps in dilute solution a constant potential must be considered. As an initial step a quantum chemical COSMO calculation for all molecules is performed and the results are stored in a database. In a separate step COSMO-RS uses the stored COSMO results to calculate the chemical potential of the molecules in a liquid solvent or mixture. The resulting chemical potentials are the basis for other thermodynamic equilibrium properties such as activity coefficients, solubility, partition coefficients, vapor pressure and free energy of solvation. The method was developed to provide a general prediction method with no need for system specific adjustment.







