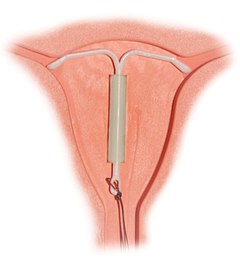
Intrauterine device (IUD) with copper, also known as intrauterine coil, is a type of intrauterine device which contains copper. It is used for birth control and emergency contraception within five days of unprotected sex. It is one of the most effective forms of birth control with a one-year failure rate around 0.7%. The device is placed in the uterus and lasts up to twelve years. It may be used by women of all ages regardless of whether or not they have had children. Following removal, fertility quickly returns.

Emergency contraception (EC) is a birth control measure, used after sexual intercourse to prevent pregnancy.

The combined oral contraceptive pill (COCP), often referred to as the birth control pill or colloquially as "the pill", is a type of birth control that is designed to be taken orally by women. It includes a combination of an estrogen and a progestogen. When taken correctly, it alters the menstrual cycle to eliminate ovulation and prevent pregnancy.

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
Heavy menstrual bleeding, previously known as menorrhagia, is a menstrual period with excessively heavy flow. It is a type of abnormal uterine bleeding (AUB).

Levonorgestrel is a hormonal medication which is used in a number of birth control methods. It is combined with an estrogen to make combination birth control pills. As an emergency birth control, sold under the brand name Plan B among others, it is useful within 72 hours. This should not be confused with EllaOne which can be effective within 120 hours of unprotected sex. The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred. It decreases the chances of pregnancy by 57 to 93%. In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy. A levonorgestrel-releasing implant is also available in some countries.

Adenomyosis is a medical condition characterized by the growth of cells that proliferate on the inside of the uterus (endometrium) atypically located among the cells of the uterine wall (myometrium), as a result, thickening of the uterus occurs. As well as being misplaced in patients with this condition, endometrial tissue is completely functional. The tissue thickens, sheds and bleeds during every menstrual cycle.
Progestogen-only pills or progestin-only pills (POP) are contraceptive pills that contain only synthetic progestogens (progestins) and do not contain estrogen. They are colloquially known as mini pills.

Vaginal rings are polymeric drug delivery devices designed to provide controlled release of drugs for intravaginal administration over extended periods of time. The ring is inserted into the vagina and provides contraception protection. Vaginal rings come in one size that fits most women.
Hormonal contraception refers to birth control methods that act on the endocrine system. Almost all methods are composed of steroid hormones, although in India one selective estrogen receptor modulator is marketed as a contraceptive. The original hormonal method—the combined oral contraceptive pill—was first marketed as a contraceptive in 1960. In the ensuing decades many other delivery methods have been developed, although the oral and injectable methods are by far the most popular. Hormonal contraception is highly effective: when taken on the prescribed schedule, users of steroid hormone methods experience pregnancy rates of less than 1% per year. Perfect-use pregnancy rates for most hormonal contraceptives are usually around the 0.3% rate or less. Currently available methods can only be used by women; the development of a male hormonal contraceptive is an active research area.
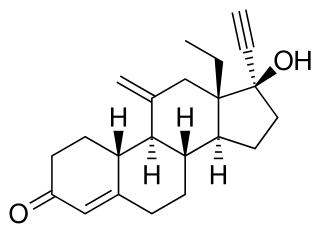
Etonogestrel is a medication which is used as a means of birth control for women. It is available as an implant placed under the skin of the upper arm under the brand names Nexplanon and Implanon, and in combination with ethinylestradiol, an estrogen, as a vaginal ring under the brand names NuvaRing and Circlet. Etonogestrel is effective as a means of birth control within 8 hours of insertion and lasts at least three or four years with some data showing effectiveness for five years. Following removal, fertility quickly returns.
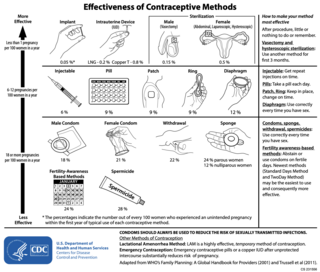
There are many methods of birth control. They vary in what is required of the user, side effects, and effectiveness. No method of birth control is ideal for every user. Outlined here are the different types of barrier methods, hormonal methods, various methods including spermicides, emergency contraceptives, and surgical methods.
Long-acting reversible contraceptives (LARC) are methods of birth control that provide effective contraception for an extended period without requiring user action. They include injections, intrauterine devices (IUDs), and subdermal contraceptive implants. They are the most effective reversible methods of contraception because their efficacy is not reliant on patient compliance. The typical use failure rates of IUDs and implants, less than 1% per year, are about the same as perfect use failure rates.
Progestogen-only contraception relies on progestogens alone to achieve contraception. It is one of the two major types of hormonal contraception, with the other major type being combined hormonal contraceptive methods. There are several progestogen only contraceptive methods:

A contraceptive implant is an implantable medical device used for the purpose of birth control. The implant may depend on the timed release of hormones to hinder ovulation or sperm development, the ability of copper to act as a natural spermicide within the uterus, or it may work using a non-hormonal, physical blocking mechanism. As with other contraceptives, a contraceptive implant is designed to prevent pregnancy, but it does not protect against sexually transmitted infections.

Birth control, also known as contraception, anticonception, and fertility control, is a method or device used to prevent pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth control only became available in the 20th century. Planning, making available, and using birth control is called family planning. Some cultures limit or discourage access to birth control because they consider it to be morally, religiously, or politically undesirable. The term birth control is a bit of a misnomer since abortion is not regularly considered under the term.

An intrauterine device (IUD), also known as intrauterine contraceptive device or coil, is a small, often T-shaped birth control device that is inserted into the uterus to prevent pregnancy. IUDs are one form of long-acting reversible birth control (LARC). One study found that female family planning providers choose LARC methods more often (41.7%) than the general public (12.1%). Among birth control methods, IUDs, along with other contraceptive implants, result in the greatest satisfaction among users.
Women's reproductive health in the United States refers to the set of physical, mental, and social issues related to the health of women in the United States. It includes the rights of women in the United States to adequate sexual health, available contraception methods, and treatment for sexually transmitted diseases. The prevalence of women's health issues in American culture is inspired by second-wave feminism in the United States. As a result of this movement, women of the United States began to question the largely male-dominated health care system and demanded a right to information on issues regarding their physiology and anatomy. The U.S. government has made significant strides to propose solutions, like creating the Women's Health Initiative through the Office of Research on Women's Health in 1991. However, many issues still exist related to the accessibility of reproductive healthcare as well as the stigma and controversy attached to sexual health, contraception, and sexually transmitted diseases.
There are many types of contraceptive methods available in France. All contraceptives are obtained by medical prescription after a visit to the family planning, a gynecologist or a midwife. With the exception of emergency contraception that does not require a prescription and can be obtained directly in a pharmacy.
Menstrual suppression refers to the practice of using hormonal management to stop or reduce menstrual bleeding. In contrast to surgical options for this purpose, such as hysterectomy or endometrial ablation, hormonal methods to manipulate menstruation are reversible.