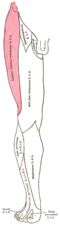
The sciatic nerve, also called the ischiadic nerve, is a large nerve in humans and other vertebrate animals which is the largest branch of the sacral plexus and runs alongside the hip joint and down the lower limb. It is the longest and widest single nerve in the human body, going from the top of the leg to the foot on the posterior aspect. The sciatic nerve has no cutaneous branches for the thigh. This nerve provides the connection to the nervous system for the skin of the lateral leg and the whole foot, the muscles of the back of the thigh, and those of the leg and foot. It is derived from spinal nerves L4 to S3. It contains fibers from both the anterior and posterior divisions of the lumbosacral plexus.

Spondylosis is the degeneration of the vertebral column from any cause. In the more narrow sense it refers to spinal osteoarthritis, the age-related degeneration of the spinal column, which is the most common cause of spondylosis. The degenerative process in osteoarthritis chiefly affects the vertebral bodies, the neural foramina and the facet joints. If severe, it may cause pressure on the spinal cord or nerve roots with subsequent sensory or motor disturbances, such as pain, paresthesia, imbalance, and muscle weakness in the limbs.

Cauda equina syndrome (CES) is a condition that occurs when the bundle of nerves below the end of the spinal cord known as the cauda equina is damaged. Signs and symptoms include low back pain, pain that radiates down the leg, numbness around the anus, and loss of bowel or bladder control. Onset may be rapid or gradual.
Nerve block or regional nerve blockade is any deliberate interruption of signals traveling along a nerve, often for the purpose of pain relief. Local anesthetic nerve block is a short-term block, usually lasting hours or days, involving the injection of an anesthetic, a corticosteroid, and other agents onto or near a nerve. Neurolytic block, the deliberate temporary degeneration of nerve fibers through the application of chemicals, heat, or freezing, produces a block that may persist for weeks, months, or indefinitely. Neurectomy, the cutting through or removal of a nerve or a section of a nerve, usually produces a permanent block. Because neurectomy of a sensory nerve is often followed, months later, by the emergence of new, more intense pain, sensory nerve neurectomy is rarely performed.

The iliopsoas muscle refers to the joined psoas major and the iliacus muscles. The two muscles are separate in the abdomen, but usually merge in the thigh. They are usually given the common name iliopsoas. The iliopsoas muscle joins to the femur at the lesser trochanter. It acts as the strongest flexor of the hip.

The femoral nerve is a nerve in the thigh that supplies skin on the upper thigh and inner leg, and the muscles that extend the knee. It is the largest branch of the lumbar plexus.

The lateral cutaneous nerve of the thigh is a cutaneous nerve of the thigh. It originates from the dorsal divisions of the second and third lumbar nerves from the lumbar plexus. It passes under the inguinal ligament to reach the thigh. It supplies sensation to the skin on the lateral part of the thigh by an anterior branch and a posterior branch.
A neurectomy, or nerve resection is a neurosurgical procedure in which a peripheral nerve is cut or removed to alleviate neuropathic pain or permanently disable some function of a nerve. The nerve is not intended to grow back. For chronic pain it may be an alternative to a failed nerve decompression when the target nerve has no motor function and numbness is acceptable. Neurectomies have also been used to permanently block autonomic function, and special sensory function not related to pain.

A spinal disc herniation is an injury to the intervertebral disc between two spinal vertebrae, usually caused by excessive strain or trauma to the spine. It may result in back pain, pain or sensation in different parts of the body, and physical disability. The most conclusive diagnostic tool for disc herniation is MRI, and treatment may range from painkillers to surgery. Protection from disc herniation is best provided by core strength and an awareness of body mechanics including good posture.
The saphenous nerve is the largest cutaneous branch of the femoral nerve. It is derived from the lumbar plexus (L3-L4). It is a strictly sensory nerve, and has no motor function. It commences in the proximal (upper) thigh and travels along the adductor canal. Upon exiting the adductor canal, the saphenous nerve terminates by splitting into two terminal branches: the sartorial nerve, and the infrapatellar nerve. The saphenous nerve is responsible for providing sensory innervation to the skin of the anteromedial leg.

Vladimir Karlovich Roth, sometimes Rot was a Russian Empire neuropathologist.

Martin Bernhardt was a German neuropathologist.
In medicine, Carnett's sign is a finding on clinical examination in which (acute) abdominal pain remains unchanged or increases when the muscles of the abdominal wall are tensed. For this part of the abdominal examination, the patient can be asked to lift the head and shoulders from the examination table to tense the abdominal muscles. An alternative is to ask the patient to raise both legs with straight knees.

Nerve compression syndrome, or compression neuropathy, or nerve entrapment syndrome, is a medical condition caused by chronic, direct pressure on a peripheral nerve. It is known colloquially as a trapped nerve, though this may also refer to nerve root compression. Its symptoms include pain, tingling, numbness and muscle weakness. The symptoms affect just one particular part of the body, depending on which nerve is affected. The diagnosis is largely clinical and can be confirmed with diagnostic nerve blocks. Occasionally imaging and electrophysiology studies aid in the diagnosis. Timely diagnosis is important as untreated chronic nerve compression may cause permanent damage. A surgical nerve decompression can relieve pressure on the nerve but cannot always reverse the physiological changes that occurred before treatment. Nerve injury by a single episode of physical trauma is in one sense an acute compression neuropathy but is not usually included under this heading, as chronic compression takes a unique pathophysiological course.

Spinal stenosis is an abnormal narrowing of the spinal canal or neural foramen that results in pressure on the spinal cord or nerve roots. Symptoms may include pain, numbness, or weakness in the arms or legs. Symptoms are typically gradual in onset and improve with leaning forward. Severe symptoms may include loss of bladder control, loss of bowel control, or sexual dysfunction.

Anterior cutaneous nerve entrapment syndrome (ACNES) is a nerve entrapment condition that causes chronic pain of the abdominal wall. It occurs when nerve endings of the lower thoracic intercostal nerves (7–12) are 'entrapped' in abdominal muscles, causing a severe localized nerve (neuropathic) pain that is usually experienced at the front of the abdomen.
Femoral nerve dysfunction, also known as femoral neuropathy, is a rare type of peripheral nervous system disorder that arises from damage to nerves, specifically the femoral nerve. Given the location of the femoral nerve, indications of dysfunction are centered around the lack of mobility and sensation in lower parts of the legs. The causes of such neuropathy can stem from both direct and indirect injuries, pressures and diseases. Physical examinations are usually first carried out, depending on the high severity of the injury. In the cases of patients with hemorrhage, imaging techniques are used before any physical examination. Another diagnostic method, electrodiagnostic studies, are recognized as the gold standard that is used to confirm the injury of the femoral nerve. After diagnosis, different treatment methods are provided to the patients depending upon their symptoms in order to effectively target the underlying causes. Currently, femoral neuropathy is highly underdiagnosed and its precedent medical history is not well documented worldwide.
A nerve decompression is a neurosurgical procedure to relieve chronic, direct pressure on a nerve to treat nerve entrapment, a pain syndrome characterized by severe chronic pain and muscle weakness. In this way a nerve decompression targets the underlying pathophysiology of the syndrome and is considered a first-line surgical treatment option for peripheral nerve pain. Despite treating the underlying cause of the disease, the symptoms may not be fully reversible as delays in diagnosis can allow permanent damage to occur to the nerve and surrounding microvasculature. Traditionally only nerves accessible with open surgery have been good candidates, however innovations in laparoscopy and nerve-sparing techniques made nearly all nerves in the body good candidates, as surgical access is no longer a barrier.
Deep gluteal syndrome describes the non-discogenic extrapelvic entrapment of the sciatic nerve in the deep gluteal space. It is an extension of non-discogenic sciatic nerve entrapment beyond the traditional model of piriformis syndrome. Symptoms are pain or dysthesias the buttocks, hip, and posterior thigh with or without radiating leg pain. Patients often report pain when sitting. The two most common causes are piriformis syndrome and fibrovascular bands, but many other causes exist. Diagnosis is usually done through physical examination, magnetic resonance imaging, magnetic resonance neurography, and diagnostic nerve blocks. Surgical treatment is an endoscopic sciatic nerve decompression.

Nerve entrapment involves a cascade of physiological changes caused by compression and tension. Some of these changes are irreversible. The magnitude and duration of the forces determines the extent of injury. In the acute form, mechanical injury and metabolic blocks impede nerve function. In the chronic form, there is a sequence of changes starting with a breakdown of the blood-nerve-barrier, followed by edema with connective tissue changes, followed by diffuse demyelination, and finally followed by axonmetesis. The injury will often be a mixed lesion where mild/moderate compression is a combination of a metabolic block and neuropraxia, while severe compression combines elements of neuropraxia and axonmetesis.