Optic neuritis describes any condition that causes inflammation of the optic nerve; it may be associated with demyelinating diseases, or infectious or inflammatory processes.

Glaucoma is a group of eye diseases that lead to damage of the optic nerve, which is important for transmitting visual information from the eye to the brain. This damage is often caused by increased pressure within the eye, known as intraocular pressure (IOP) and may cause vision loss if left untreated. The word glaucoma originated from the Greek word ΓλαύV̇ξ (glaukos), which means "to glow". Glaucoma has been called the "silent thief of sight" because the loss of vision usually occurs slowly over a long period of time. It is associated with old age, a family history of glaucoma, and certain medical conditions or medications.

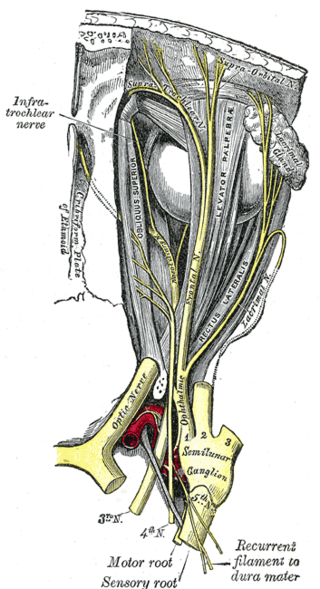
In neuroanatomy, the optic nerve, also known as the second cranial nerve, cranial nerve II, or simply CN II, is a paired cranial nerve that transmits visual information from the retina to the brain. In humans, the optic nerve is derived from optic stalks during the seventh week of development and is composed of retinal ganglion cell axons and glial cells; it extends from the optic disc to the optic chiasma and continues as the optic tract to the lateral geniculate nucleus, pretectal nuclei, and superior colliculus.
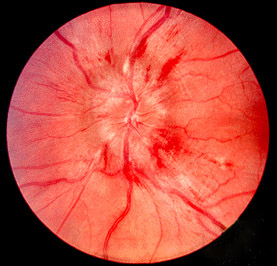
Papilledema or papilloedema is optic disc swelling that is caused by increased intracranial pressure due to any cause. The swelling is usually bilateral and can occur over a period of hours to weeks. Unilateral presentation is extremely rare.
The National Eye Institute (NEI) is part of the U.S. National Institutes of Health (NIH), an agency of the U.S. Department of Health and Human Services. The mission of NEI is "to eliminate vision loss and improve quality of life through vision research." NEI consists of two major branches for research: an extramural branch that funds studies outside NIH and an intramural branch that funds research on the NIH campus in Bethesda, Maryland. Most of the NEI budget funds extramural research.

Amaurosis fugax is a painless temporary loss of vision in one or both eyes.

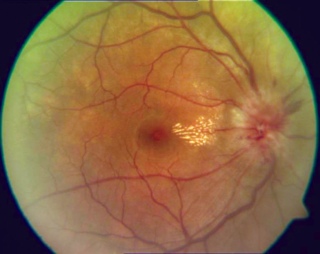
The optic disc or optic nerve head is the point of exit for ganglion cell axons leaving the eye. Because there are no rods or cones overlying the optic disc, it corresponds to a small blind spot in each eye.
Anterior ischemic optic neuropathy (AION) is a medical condition involving loss of vision caused by damage to the optic nerve as a result of insufficient blood supply (ischemia). This form of ischemic optic neuropathy is generally categorized as two types: arteritic AION, in which the loss of vision is the result of an inflammatory disease of arteries in the head called temporal arteritis, and non-arteritic AION, which is due to non-inflammatory disease of small blood vessels.
Ischemic optic neuropathy (ION) is the loss of structure and function of a portion of the optic nerve due to obstruction of blood flow to the nerve. Ischemic forms of optic neuropathy are typically classified as either anterior ischemic optic neuropathy or posterior ischemic optic neuropathy according to the part of the optic nerve that is affected. People affected will often complain of a loss of visual acuity and a visual field, the latter of which is usually in the superior or inferior field.
Arteritic anterior ischemic optic neuropathy is the cause of vision loss that occurs in temporal arteritis. Temporal arteritis is an inflammatory disease of medium-sized blood vessels that happens especially with advancing age. AAION occurs in about 15-20 percent of patients with temporal arteritis. Damage to the blood vessels supplying the optic nerves leads to insufficient blood supply (ischemia) to the nerve and subsequent optic nerve fiber death. Most cases of AAION result in nearly complete vision loss first to one eye. If the temporal arteritis is left untreated, the fellow eye will likely suffer vision loss as well within 1–2 weeks. Arteritic AION falls under the general category of anterior ischemic optic neuropathy, which also includes non-arteritic AION. AION is considered an eye emergency, immediate treatment is essential to rescue remaining vision.
Toxic and nutritional optic neuropathy is a group of medical disorders defined by visual impairment due to optic nerve damage secondary to a toxic substance and/or nutritional deficiency. The causes of these disorders are various, but they are linked by shared signs and symptoms, which this article will describe. In several of these disorders, both toxic and nutritional factors play a role, acting synergistically.
Optic neuropathy is damage to the optic nerve from any cause. The optic nerve is a bundle of millions of fibers in the retina that sends visual signals to the brain. [1].
Ocular ischemic syndrome is the constellation of ocular signs and symptoms secondary to severe, chronic arterial hypoperfusion to the eye. Amaurosis fugax is a form of acute vision loss caused by reduced blood flow to the eye; it may be a warning sign of an impending stroke, as both stroke and retinal artery occlusion can be caused by thromboembolism due to atherosclerosis elsewhere in the body. Consequently, those with transient blurring of vision are advised to urgently seek medical attention for a thorough evaluation of the carotid artery. Anterior segment ischemic syndrome is a similar ischemic condition of anterior segment usually seen in post-surgical cases. Retinal artery occlusion leads to rapid death of retinal cells, thereby resulting in severe loss of vision.

Optic disc drusen (ODD) are globules of mucoproteins and mucopolysaccharides that progressively calcify in the optic disc. They are thought to be the remnants of the axonal transport system of degenerated retinal ganglion cells. ODD have also been referred to as congenitally elevated or anomalous discs, pseudopapilledema, pseudoneuritis, buried disc drusen, and disc hyaline bodies.
Central retinal artery occlusion (CRAO) is a disease of the eye where the flow of blood through the central retinal artery is blocked (occluded). There are several different causes of this occlusion; the most common is carotid artery atherosclerosis.

Blurred vision is an ocular symptom where vision becomes less precise and there is added difficulty to resolve fine details.
Autoimmune optic neuropathy (AON), sometimes called autoimmune optic neuritis, may be a forme fruste of systemic lupus erythematosus (SLE) associated optic neuropathy. AON is more than the presence of any optic neuritis in a patient with an autoimmune process, as it describes a relatively specific clinical syndrome. AON is characterized by chronically progressive or recurrent vision loss associated with serological evidence of autoimmunity. Specifically, this term has been suggested for cases of optic neuritis with serological evidence of vasculitis by positive ANA, despite the lack of meeting criteria for SLE. The clinical manifestations include progressive vision loss that tends to be steroid-responsive and steroid dependent.
Mitohondrial optic neuropathies are a heterogenous group of disorders that present with visual disturbances resultant from mitochondrial dysfunction within the anatomy of the Retinal Ganglion Cells (RGC), optic nerve, optic chiasm, and optic tract. These disturbances are multifactorial, their aetiology consisting of metabolic and/or structural damage as a consequence of genetic mutations, environmental stressors, or both. The three most common neuro-ophthalmic abnormalities seen in mitochondrial disorders are bilateral optic neuropathy, ophthalmoplegia with ptosis, and pigmentary retinopathy.
Sickle cell retinopathy can be defined as retinal changes due to blood vessel damage in the eye of a person with a background of sickle cell disease. It can likely progress to loss of vision in late stages due to vitreous hemorrhage or retinal detachment. Sickle cell disease is a structural red blood cell disorder leading to consequences in multiple systems. It is characterized by chronic red blood cell destruction, vascular injury, and tissue ischemia causing damage to the brain, eyes, heart, lungs, kidneys, spleen, and musculoskeletal system.
Diabetic papillopathy is an ocular complication of diabetes mellitus characterized by optic disc swelling and edema of optic nerve head. The condition may affect both type 1 and type 2 diabetic patients.








