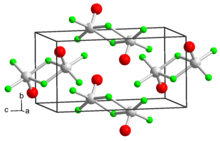
Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque crystalline compound is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Vanadium(II) chloride is the inorganic compound with the formula VCl2, and is the most reduced vanadium chloride. Vanadium(II) chloride is an apple-green solid that dissolves in water to give purple solutions.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.

Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
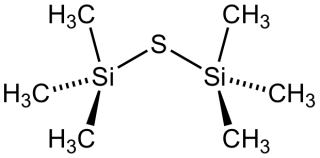
Bis(trimethylsilyl) sulfide is the chemical compound with the formula ((CH3)3Si)2S. Often abbreviated (tms)2S, this colourless, vile-smelling liquid is a useful aprotic source of "S2−" in chemical synthesis.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal M with anionic bis(trimethylsilyl)amide ligands (the −N 2 monovalent anion, or −N 2 monovalent group, and are part of a broader category of metal amides.
Organoxenon chemistry is the study of the properties of organoxenon compounds, which contain carbon to xenon chemical bonds. The first organoxenon compounds were divalent, such as (C6F5)2Xe. The first tetravalent organoxenon compound, [C6F5XeF2][BF4], was synthesized in 2004. So far, more than one hundred organoxenon compounds have been researched.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Bis(pentafluorophenyl)xenon is an unstable organic compound of xenon. It consists of two fluorinated phenyl rings connected to xenon.

Fluorosulfite is an ion with the formula SO2F−. The term is also used for compounds or salts containing this group. Fluorosulfite was discovered in 1953 by F Seel and H Meier.

Molybdenum oxytetrafluoride is the inorganic compound with the formula MoOF4. It is a white, diamagnetic solid. According to X-ray crystallography, it is a coordination polymer consisting of a linear chain of alternating Mo and F atoms. Each Mo center is octahedral, the coordination sphere being defined by oxide, three terminal fluorides, and two bridging fluorides. In contrast to this motif, tungsten oxytetrafluoride crystallizes as a tetramer, again with bridging fluoride ligands.

Vanadium dioxide fluoride is the inorganic compound with the formula VO2F. It is an orange diamagnetic solid. The compound adopts the same structure as iron(III) fluoride, with octahedral metal centers and doubly bridging oxide and fluoride ligands. It is prepared by the reaction of vanadium pentoxide and vanadium(V) oxytrifluoride: