
Europium is a chemical element; it has symbol Eu and atomic number 63. Europium is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. It is the most chemically reactive, least dense, and softest of the lanthanide elements. It is soft enough to be cut with a knife. Europium was isolated in 1901 and named after the continent of Europe. Europium usually assumes the oxidation state +3, like other members of the lanthanide series, but compounds having oxidation state +2 are also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.

Gadolinium is a chemical element; it has symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is a malleable and ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of 20 °C (68 °F) is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties.

Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.

Terbium is a chemical element; it has the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.

Group 3 is the first group of transition metals in the periodic table. This group is closely related to the rare-earth elements. It contains the four elements scandium (Sc), yttrium (Y), lutetium (Lu), and lawrencium (Lr). The group is also called the scandium group or scandium family after its lightest member.

Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula Y F3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY(F,Cl)6. Sometimes mineral fluorite contains admixtures of yttrium.
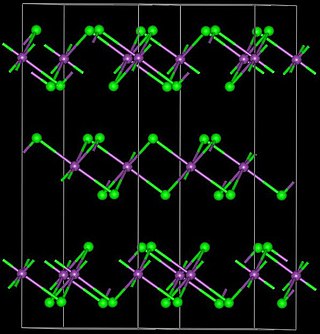
Yttrium(III) bromide is an inorganic compound with the chemical formula YBr3. It is a white solid. Anhydrous yttrium(III) bromide can be produced by reacting yttrium oxide or yttrium(III) bromide hydrate and ammonium bromide. The reaction proceeds via the intermediate (NH4)3YBr6. Another method is to react yttrium carbide (YC2) and elemental bromine. Yttrium(III) bromide can be reduced by yttrium metal to YBr or Y2Br3. It can react with osmium to produce Y4Br4Os.

Yttrium(III) antimonide (YSb) is an inorganic chemical compound.

Yttrium arsenide is an inorganic compound of yttrium and arsenic with the chemical formula YAs. It can be prepared by reacting yttrium and arsenic at high temperature. Some literature has done research on the eutectic system of it and zinc arsenide.

Yttrium is a chemical element; it has symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. 89Y is the only stable isotope and the only isotope found in the Earth's crust.

Yttrium phosphide is an inorganic compound of yttrium and phosphorus with the chemical formula YP. The compound may be also classified as yttrium(III) phosphide.
Yttrium(III) sulfate is an inorganic compound with the formula Y2(SO4)3. The most common form is the anhydrate and octahydrate.

Yttrium(III) nitrate is an inorganic compound, a salt with the formula Y(NO3)3. The hexahydrate is the most common form commercially available.
An yttrium compound is a chemical compound containing yttrium. Among these compounds, yttrium generally has a +3 valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in water, but soluble in excess oxalate or carbonate solutions as complexes are formed. Sulfates and double sulfates are generally soluble. They resemble the "yttrium group" of heavy lanthanide elements.
Thullium(III) fluoride is an inorganic compound with the chemical formula TmF3.
Yttrium(III) hydroxide is an inorganic compound and an alkali with the chemical formula Y(OH)3.
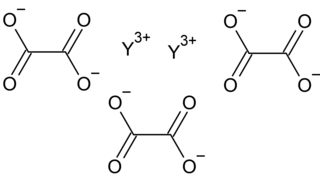
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.
Yttrium iodide is a binary inorganic compound, a salt of yttrium and hydroiodic acid with the formula YI
3. The compound forms colorless crystals, soluble in water.
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.












