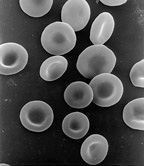
Red blood cells (RBCs), scientific name erythrocytes (from ancient Greek erythros 'red' and kytos 'hollow vessel', with -cyte translated as 'cell' in modern usage), also known as red cells, erythroid cells, and rarely haematids, are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. Erythrocytes take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries.

Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, acute mountain sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure and to alkalinize urine. It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out. It is taken by mouth or injection into a vein. Acetazolamide is a first generation carbonic anhydrase inhibitor and it decreases the ocular fluid and osmolality in the eye to decrease intraocular pressure.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

An antiporter is an integral membrane protein involved in secondary active transport. It is a type of cotransporter, which means that uses the movement of one In the case of an antiporter, two or more different molecules or ions are moved across a phospholipid membrane, such as the plasma membrane, in opposite directions, one into the cell and one out of the cell. This is in contrast to symporters, which are another type of cotransporter that moves two or more ions in the same direction.

Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They enable coupled or cotransport and include antiporters and symporters. In general, cotransporters consist of two out of the three classes of integral membrane proteins known as transporters that move molecules and ions across biomembranes. Uniporters are also transporters but move only one type of molecule down its concentration gradient and are not classified as cotransporters.
The Na–K–Cl cotransporter (NKCC) is a transport protein that aids in the secondary active transport of sodium, potassium, and chloride into cells. In humans there are two isoforms of this membrane transport protein, NKCC1 and NKCC2, encoded by two different genes. Two isoforms of the NKCC1/Slc12a2 gene result from keeping or skipping exon 21 in the final gene product.
Alkaline tide (mal del puerco) refers to a condition, normally encountered after eating a meal, where during the production of hydrochloric acid by the parietal cells in the stomach, the parietal cells secrete bicarbonate ions across their basolateral membranes and into the blood, causing a temporary increase in blood pH.
Pendrin is an anion exchange protein that in humans is encoded by the SLC26A4 gene . Pendrin was initially identified as a sodium-independent chloride-iodide exchanger with subsequent studies showing that it also accepts formate and bicarbonate as substrates. Pendrin is similar to the Band 3 transport protein found in red blood cells. Pendrin is the protein which is mutated in Pendred syndrome, which is an autosomal recessive disorder characterized by sensorineural hearing loss, goiter and a partial organification problem detectable by a positive perchlorate test.

Protein 4.1,, is a protein associated with the cytoskeleton that in humans is encoded by the EPB41 gene. Protein 4.1 is a major structural element of the erythrocyte membrane skeleton. It plays a key role in regulating membrane physical properties of mechanical stability and deformability by stabilizing spectrin-actin interaction. Protein 4.1 interacts with spectrin and short actin filaments to form the erythrocyte membrane skeleton. Mutations of spectrin and protein 4.1 are associated with elliptocytosis or spherocytosis and anemia of varying severity.

Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a cardiovascular system and refers to the exchange of bicarbonate (HCO3−) and chloride (Cl−) across the membrane of red blood cells (RBCs).
Carbonic anhydrase II is one of sixteen forms of human α carbonic anhydrases. Carbonic anhydrase catalyzes reversible hydration of carbon dioxide. Defects in this enzyme are associated with osteopetrosis and renal tubular acidosis. Renal carbonic anhydrase allows the reabsorption of bicarbonate ions in the proximal tubule. Loss of carbonic anhydrase activity in bones impairs the ability of osteoclasts to promote bone resorption, leading to osteopetrosis.

Electrogenic sodium bicarbonate cotransporter 1, sodium bicarbonate cotransporter is a membrane transport protein that in humans is encoded by the SLC4A4 gene.

Anion exchange protein 2 (AE2) is a membrane transport protein that in humans is encoded by the SLC4A2 gene. AE2 is functionally similar to the Band 3 Cl−/HCO3− exchange protein.

Carbonic anhydrase 4 is an enzyme that in humans is encoded by the CA4 gene.

Chloride anion exchanger, also known as down-regulated in adenoma, is a protein that in humans is encoded by the SLC26A3 gene.

Carbonic anhydrase 12 is an enzyme that in humans is encoded by the CA12 gene.

Anion exchange protein 3 is a membrane transport protein that in humans is encoded by the SLC4A3 gene. AE3 is functionally similar to the Band 3 Cl−/HCO3− exchange protein but it is expressed primarily in brain neurons and in the heart. Like AE2 its activity is sensitive to pH. AE3 mutations have been linked to seizures.

Anion exchange transporter is a protein that in humans is encoded by the SLC26A7 gene.

The carbonic anhydrases form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid. The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.
The anion exchanger family is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria. Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.